Abstract
Cortical information storage requires combined changes in connectivity and synaptic strength between neurons, but the signaling mechanisms underlying this two-step wiring plasticity are unknown. Because acute 17β-estradiol (E2) modulates cortical memory, we examined its effects on spine morphogenesis, AMPA receptor trafficking, and GTPase signaling in cortical neurons. Acute E2 application resulted in a rapid, transient increase in spine density, accompanied by temporary formation of silent synapses through reduced surface GluR1. These rapid effects of E2 were dependent on a Rap/AF-6/ERK1/2 pathway. Intriguingly, NMDA receptor (NMDAR) activation after E2 treatment potentiated silent synapses and elevated spine density for as long as 24 h. Hence, we show that E2 transiently increases neuronal connectivity by inducing dynamic nascent spines that “sample” the surrounding neuropil and that subsequent NMDAR activity is sufficient to stabilize or “hold” E2-mediated effects. This work describes a form of two-step wiring plasticity relevant for cortical memory and identifies targets that may facilitate recovery from brain injuries.
Information storage in the cortex is thought to involve neurons making transient contacts with neighboring cells. In vivo studies have shown that these contacts can persist after experience-dependent stimuli (1–3). This two-step form of wiring plasticity has been referred to as a “sample-and-hold” theory (2). Such alteration in synapse number may result in increased and stronger connections between neurons and thus may represent a mechanism that features enormous capacity for cortical information storage (1, 3). Cellularly, changes in dendritic spine number and morphology are key components of synaptic plasticity and circuit rewiring. Concurrent changes in dendritic spine morphology and in NMDA receptor (NMDAR) and AMPA receptor (AMPAR) content at synapses are required for functional plasticity (4–6). These parallel changes in function and morphology associated with plasticity are dependent on small GTPase signaling (6, 7), but the cellular and molecular underpinnings of a sample-and-hold plasticity remain unclear.
Neuromodulation of synaptic plasticity is thought to influence information storage. Evidence for the presence of multiple enzymes in the brain that control neurosteroid biosynthesis has been described in male and female subjects (8), and recent evidence has implicated 17β-estradiol (E2) as a neuromodulator (9). The neuromodulatory role of E2 is thought to occur through local production of the steroid in specific brain areas, including the cortex (8, 9). Local production of E2 results in a much higher concentration than that of the circulating hormone and is required for rapid, nongenomic actions of the steroid in the brain (9, 10). Recent studies have shown that E2 rapidly affects synaptic plasticity (11, 12) and cortical memory (13–15) in a temporally specific manner, but as of yet, the molecular and cellular mechanisms that underlie the rapid effects of E2 in the cortex remain unresolved. However, the rapid and transient effects of this steroid on both synaptic plasticity and cognition establish E2 as a good candidate to modulate wiring plasticity.
Here we report a form for two-step wiring plasticity whereby E2-induced increases in synaptic connectivity are sustained by subsequent NMDAR activation. These findings reveal a mechanism that likely underlies wiring plasticity that may be important for cortical function and recovery from brain injury.
E2 Rapidly and Transiently Modulates Synaptogenesis.
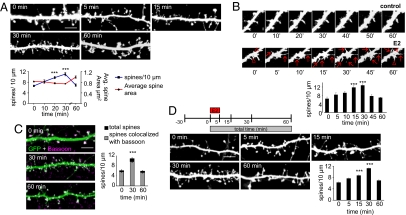
Several in vivo studies have demonstrated that acute (<1 h) intracortical or systemic E2 injections result in enhanced performance in tasks reliant on cortical processing (13–15). These effects are thought to be dependent on E2 modulation of synaptic function and dendritic spine structure (11, 12). However, the signaling pathway(s) that control E2-mediated changes in dendritic spine morphology have not been described. Moreover, spine density changes are thought to contribute to modified connectivity between neurons (1, 2). To investigate whether E2 could rapidly (≤1 h) increase the connectivity of cortical neurons, we examined the dendritic spine morphology in mature (28 days in vitro) cultured cortical neurons, expressing GFP, treated with 10 nM E2 for a maximum of 60 min. After E2 treatment, spine density peaked at 30 min and returned to control levels at 60 min (0 min, 6.6 ± 0.32 spines per 10 μm; 5 min, 8.2 ± 0.62 spines per 10 μm; 15 min, 9.8 ± 0.45 spines per 10 μm; 30 min, 11.0 ± 0.63 spines per 10 μm; 60 min, 6.9 ± 0.49 spines per 10 μm) (Fig. 1A). Accompanying the increase in spine numbers, the average spine area and spine breadth were significantly reduced at 30 min, suggesting that there was a greater number of thin spines present, and by 60 min, spine area and breadth returned to control levels (area: 0 min, 0.8 ± 0.02 μm2; 30 min, 0.7 ± 0.02 μm2; 60 min, 0.9 ± 0.02 μm2; breadth: 0 min, 0.97 ± 0.02 μm; 30 min, 0.88 ± 0.01 μm; 60 min, 0.99 ± 0.03 μm) [Fig. 1A and supporting information (SI) Fig. S1A). Time-lapse imaging of neurons treated with E2 for as long as 60 min revealed that the increase in spine number was a result of the rapid and transient production of thin spines; that is, the majority of newly formed spines retracted within 60 min (Fig. 1B and Movie S1). Furthermore, by examining the colocalization of spines with the presynaptic marker bassoon, we determined that ≈90% of all spines made contact presynaptically after challenge with E2 (total spines vs. spines with bassoon: 0 min, 6.1 ± 0.35 spines per 10 μm vs. 5.6 ± 0.43 spines per 10 μm; 30 min, 10.9 ± 0.35 spines per 10 μm vs. 9.7 ± 0.35 spines per 10 μm; 60 min, 5.9 ± 0.26 spines per 10 μm vs. 5.3 ± 0.29 spines per 10 μm) (Fig. 1C). Importantly, as our studies were performed in the presence of NMDAR blockade by (2R)-amino-5-phosphonovaleric acid (APV), they demonstrate that the transient actions of E2 are independent of NMDAR activity. This finding is in contrast to a recent report that demonstrated that E2-mediated increase of spine density in hippocampal neurons could be blocked by NDMAR antagonism (11, 16). The transient effects of E2 on spine density were also observed in cells not chronically treated with APV (Fig. S1B), indicating that these effects were not indirectly caused by chronic NMDAR blockade. Together these data suggest that E2 rapidly and transiently enhances connectivity by generating spines that make presynaptic contacts.
Fig. 1.
E2 rapidly and transiently enhances connectivity by increasing spine turnover. (A) EGFP-expressing cortical neurons (28 days in vitro) were treated with 10 nM E2 for 0, 5, 15, 30, and 60 min. Spine density increased transiently (blue line); average area decreased transiently (red line). (B) Time-lapse imaging of cortical neuron expressing EGFP. Cells were imaged for 60 min before treatment and then at 0, 5, 10, 15, 30, 45, and 60 min after treatment with E2. Red arrows indicate novel spines; red triangles represent persistent spines. (C) E2-treated cells were stained for bassoon. The total number of spines (black bar) and the number of spines colocalizing with bassoon (gray bar) were measured. (D) Schematic of brief, 5-min exposure of E2 experiment. Neurons were treated with E2 for 5 min (E2 pulse); cells were fixed at 0, 5, 15, 30, and 60 min from initial addition of E2. ***, P < 0.001. [Scale bars, 5 μm (A, C, and D); 1 μm (B).]
Nongenomic Mechanisms Underlie Rapid Effects of E2 on Synaptogenesis.
To determine if E2 was acting via the classical nuclear estrogen α-receptor (ERα), we used the selective ERα receptor modulator tamoxifen. Tamoxifen failed to block the rapid increase in spine density (P < 0.05) (Fig. S1C) despite its ability to block the chronic effects of E2 (16, 17). The rapid effects of E2 were protein-synthesis independent, as revealed by cycloheximide treatment (P < 0.001) (Fig. S1D) demonstrating a nongenomic mechanism of action. Furthermore, reduction of GABAergic signaling by picrotoxin was unable to mimic the rapid effects of E2 on dendritic spines (Fig. S1E), contrary to chronic actions of E2 on spines (18). As E2 can be rapidly produced and metabolized in the cortex (9), its presence is likely to be temporally restricted, characteristic of neuromodulators. Therefore, to test whether E2 can act as a neuromodulator, we treated cells with a brief application (5 min) of E2. This treatment was sufficient to produce a rapid, transient increase in spine density after 30 min (E2 pulse; P < 0.001) (Fig. 1D). As E2 has been shown to be synthesized de novo in neuronal cultures and to be important for hippocampal synapse formation (19), we examined the effect of reducing endogenous E2 levels on basal spine density. Blocking endogenous E2 synthesis with the aromatase inhibitor androstatrienedione resulted in reduced basal spine density (Fig. S1F), confirming that E2 acts as a neuromodulator. Collectively, these data suggest that rapid E2 effects are mediated via a nongenomic mechanism and that E2 can act as a neuromodulator.
Rap/AF-6/ERK1/2 Signaling Cascade Underlies E2 Effects on Synaptogenesis.
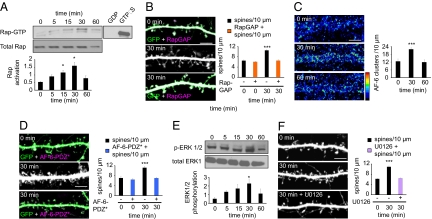
A number of second messenger systems have been implicated in rapid E2 signaling in the brain (10), but how E2 rapidly affects spines has not been determined. In nonneuronal cells, E2 has been shown to influence small GTPase activity and subsequently alter actin dynamics (20). In neurons, small GTPases, specifically Rap and Rac, are critical for synaptogenesis and GluR1 trafficking through the rearrangement of the actin cytoskeleton (6, 7). Hence, E2 signaling in neurons may require Rap or Rac activation. E2-induced synaptogenesis depended on Rap activity, because treatment resulted in a time-dependent activation of Rap (P < 0.05) (Fig. 2A). Inhibition of Rap activity by overexpression of RapGAP (7) blocked E2-induced synaptogenesis (P < 0.001) (Fig. 2B). Use of dominant negative Rap (RapN17) confirmed that E2 mediated its effects through Rap1 (Fig. S2A). In contrast, inhibition of Ras by farnesyl transferase inhibitor II did not block the transient increase in spine density induced by E2 (Fig. S2B). Interestingly, Rac activity was slightly reduced after 60 min of treatment, but the reduction was not statistically significant (Fig. S2C). To dissect how E2-mediated Rap activation was influencing spine morphogenesis, we investigated the localization of the scaffold protein AF-6, a downstream target of Rap (7). AF-6 transiently clustered upon E2 treatment (0 min, 12.6 ± 1.86 clusters per 10 μm; 30 min, 21.84 ± 1.43 clusters per 10 μm; 60 min, 11.87 ± 1.94 clusters per 10 μm) (Fig. 2C and Fig. S3A), suggesting that it may play some role in mediating E2 effects on spines. When an AF-6 mutant with a single point mutation in its PDZ domain (AF-6-PDZ*) (7) was overexpressed, E2-induced synaptogenesis was abolished (P < 0.001) (Fig. 2D), demonstrating that AF-6 was critical for E2-mediated synaptogenesis. A second target of Rap is the MAP kinase ERK1/2. When investigated, a time-dependent increase in phosphorylated ERK1/2 was observed (P < 0.05) (Fig. 2E). This finding is in agreement with previous studies that have demonstrated an E2-dependent activation of ERK1/2 (12). Inhibition of ERK1/2 activity by U0126 also blocked E2-induced synaptogenesis (P < 0.001) (Fig. 2F), implying a role for ERK1/2 in E2 signaling. Together, these data demonstrate that a Rap/AF-6/Erk1/2 signaling cascade is critical for E2-induced synaptogenesis.
Fig. 2.
Rap activity underlies E2-mediated synaptogenesis. (A) Rap activity after treatment with E2; Rap activity increases in a time-dependent manner. (B) Effect of overexpression of RapGAP on E2-induced increase in spine density; RapGAP (orange bars) blocks rapid E2 actions. (C) Effect of E2 on AF-6 clustering; AF-6 clusters in a transient manner. (D) In the presence of a mutant construct, AF-6-PDZ* (blue bars), E2 is unable to induce a spine-density increase. (E) Time course of ERK1/2 phosphorylation after E2 treatment. (F) Inhibition of ERK1/2 by U0126 (purple bar) inhibits E2-mediated increase in spine density. *, P < 0.05; ***, P < 0.001. (Scale bars, 5 μm.)
E2 Rapidly and Bidirectionally Modulates Glutamate Receptor Trafficking.
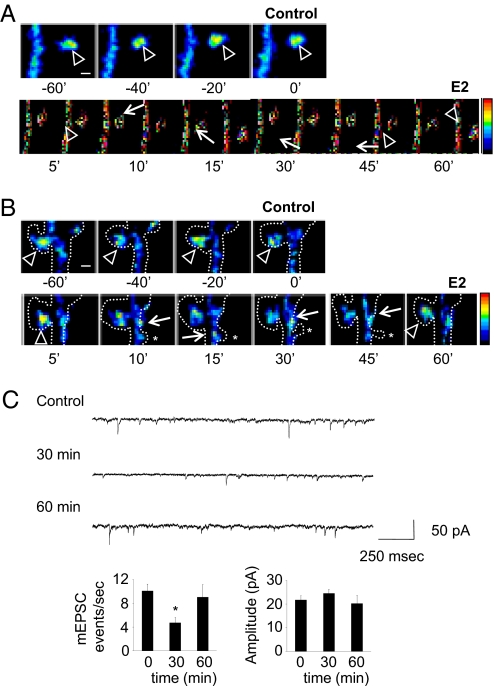
Because alterations in synaptogenesis and Rap signaling are accompanied by changes in glutamate receptor content (4, 7), we investigated whether AMPAR or NMDAR localizations changed. Surface expression of the AMPAR subunit GluR1 (n-GluR1) was transiently reduced after E2 treatment. Conversely, the NMDAR subunit NR1 puncta temporarily increased, concurrent with spine density (n-GluR1 puncta, P < 0.001; NR1 puncta, P < 0.001) (Fig. S3B). This bidirectional pattern of receptor redistribution is consistent with the generation of silent synapses (21, 22). We investigated the transient reduction in surface GluR1 by examining immunostaining of GluR1 in spines. This investigation revealed that less GluR1 was present in spines, and more GluR1 accumulated in the dendritic shaft within 30 min of E2 treatment; by 60 min, GluR1 levels in spines and dendritic shaft were similar to those of controls (Fig. S3C). GluR1 trafficking was not blocked by pretreatment with tamoxifen (Fig. S3D), suggesting that ERα was not involved in the rapid and transient trafficking of this AMPAR subunit. To determine whether the decrease in surface GluR1 was solely a result of the creation of novel spines lacking GluR1 or the redistribution of GluR1 within existing spines, we performed time-lapse imaging of GFP-GluR1. This imaging confirmed that GluR1 was transiently removed and then reinserted into existing spines but novel spines did not receive GluR1 (Fig. 3 A and B and Movie S2). Combined, these data demonstrate that E2 transiently increases synaptic NR1 and removes GluR1 from the surface into dendritic shafts, anatomically mimicking the formation of silent synapses.
Fig. 3.
E2 rapidly and transiently induces the formation of silent synapses through trafficking of GluR1 and NR1. (A and B) Time-lapse imaging of neurons expressing GFP-GluR1. Cells were imaged for 60 min before and after administration of E2. Arrowheads indicate GFP-GluR1 in spine heads; arrows indicate GFP-GluR1 in dendritic shaft. Dotted lines indicate neuron outline, as determined by Discosoma red fluorescent protein coexpression; asterisks show transient emergence of novel spines upon E2 treatment. (C) AMPAR mEPSCs after E2 treatment. Frequency and average amplitude of mEPSCs were measured; frequency, but not amplitude, of mEPSCs was significantly reduced at 30 min. *, P < 0.05; ***, P < 0.001. [Scale bars, 1 μm (A and B).]
AMPAR Transmission Is Rapidly Decreased After Acute E2 Treatment.
Because the bidirectional trafficking of NR1 and GluR1 by E2 suggests the creation of silent synapses (21, 22), these effects would likely alter AMPAR transmission. Thus, we examined AMPAR miniature excitatory postsynaptic currents (mEPSCs) in cultured neurons treated with E2. Electrophysiological recording demonstrated that E2 treatment resulted in fewer mEPSC events after 30 min of E2 treatment (frequency: 0 min, 10.1 ± 1.17 events per s; 30 min, 4.7 ± 0.9 events per s; 60 min, 9.1 ± 2.14 events per s), consistent with the generation of silent synapses (23); however, no effects on amplitude were observed (Fig. 3C). This finding is consistent with the reported generation of silent synapses by a reduction of AMPAR-containing synapses in PSD95 knockout mice (23). The bidirectional pattern of NR1 and GluR1 trafficking and the electrophysiological recordings of AMPAR transmission demonstrate that E2 rapidly yet transiently induces silent-synapse formation. It is of note that the generation of silent synapses by acute E2 treatment is independent of NMDAR activity, because these experiments were performed in the presence of NMDAR blockade. These data suggest that, concurrent with increases in connectivity, E2 forms silent synapses by increasing NR1 levels and removing GluR1 from the surface, leading to depressed AMPAR transmission.
Synaptic Activity Sustains and Potentiates E2 Effects.
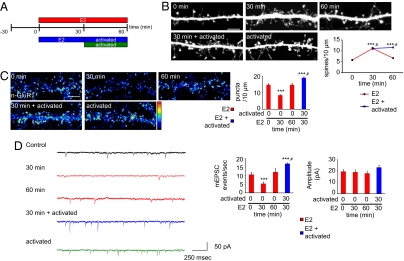
Previous in vivo studies have shown that cortical neurons make transient connections that may require a Hebbian-like mechanism to stabilize them (1–3), but the cellular and molecular components of this sample-and-hold model have remained unclear. We postulated that the transient effect on spine density and silent-synapse generation by E2 may allow neurons to sample synaptic partners in a transient manner. Hence, activity-like stimuli would sustain increased spine numbers and simultaneously potentiate silent synapses. We tested this hypothesis by activating NMDARs after E2 treatment (Fig. 4A).Indeed, NMDAR activation stabilized the E2-increased spine number: spine density was significantly greater than with E2 treatment for 60 min or NMDAR activation alone (0 min, 5.8 ± 0.33 spines per 10 μm; 30 min, 11.1 ± 0.48 spines per 10 μm; 60 min, 6.6 ± 0.23 spines per 10 μm; 30 min E2/activated, 11.5 ± 0.56 spines per 10 μm) (Fig. 4B and Fig. S4A). These effects were replicated in neurons cultured without APV, which were then briefly (2 h) treated with APV before E2 treatment and subsequent NMDAR activation (Fig. S4B), further demonstrating that these effects did not result from chronic NMDAR blockade. Furthermore, NMDAR activity led to the phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) even in the presence of E2 (Fig. S4C), consistent with activation of a CaMKII/Rac pathway (6). These data show that NMDAR activity after E2 treatment is able to sustain the transient effects of E2 on synaptogenesis.
Fig. 4.
Synaptic activity sustains and potentiates E2 effects. (A) Schematic of combined E2 and NMDAR activation experiments. Neurons were treated with E2 for 0, 30, or 60 min; for 30 min with E2 followed by activation of NMDAR for 30 min; or activation of NMDAR alone for 30 min. (B) Effect of E2 priming on dendritic morphology; cells were treated as described in A. (C) Surface staining of GluR1 (n-GluR1) after treatments as in A. The total number of n-GluR1 puncta was measured; n-GluR1 levels are significantly greater than control after combined E2 and NMDAR activity (blue bar). (D) Electrophysiological recording of AMPAR mEPSCs after treatments. Frequency of AMPAR mEPSC events are significantly greater than controls with combined E2 and NMDAR activation (blue bars). AMPAR amplitudes are also increased with treatment with E2 followed by NMDAR activation, but not significantly. **, P < 0.005; ***, P < 0.001; #, different subgroup of significance according to Tukey B post hoc analysis. (Scale bar, 5 μm.)
Coactivation of E2 and NMDARs Increases AMPAR Transmission.
Silent synapses have been observed in a number of neuronal preparations and are potentiated during plasticity through GluR1 insertion into synapses lacking this subunit (24). Therefore, to determine whether NMDAR activity after E2 treatment potentiated E2-induced silent synapses, we examined the amount of surface GluR1 after coactivation with E2 and NMDARs. NMDAR activation after E2 priming resulted in significantly higher surface GluR1 levels than in controls and after E2 treatment for 60 min (P < 0.001) (Fig. 4C and Fig. S4D). We confirmed this increase in surface expression of GluR1 by examination of GluR1 content in spines. This examination demonstrated that, after combined E2 and NMDAR activation, a greater amount of GluR1 was found in spines (Fig. S5A). Additionally, a significantly greater number of GluR1 colocalized with bassoon, suggesting that there were more functional synapses and that E2-induced silent synapses were potentiated (Fig. S5B). Indeed, analysis of AMPAR mEPSCs further demonstrated that silent synapses were potentiated. The frequency of mEPSC events were significantly increased, consistent with the formation of functional synapses (frequency: 0 min, 10.7 ± 1.23 events per sec; 30 min, 5.3 ± 0.99 events per sec; 60 min, 12.1 ± 2.01 events per sec; 30 min E2/activated, 16.9 ± 1.08 events per sec) (Fig. 4D and Fig. S5C). Amplitudes also increased upon NMDAR activation after priming with E2, but this change was not significant (P = 0.06). Together, these data suggest that NMDAR activation results in the sustained increase of synaptic connectivity and a potentiation of silent synapses induced by E2.
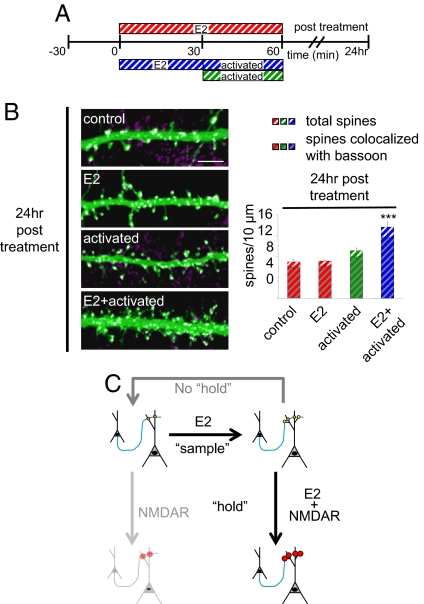
Long-Term Enhancement of Neuronal Connectivity by Coordinated E2 and NMDAR Activity.
If the observed increase in connectivity does result in an enhancement of wiring plasticity, one would expect increased spine density to persist for longer durations. Remarkably, increased spine density was still observed 24 h after stimulation with combined E2 and NMDAR treatments, suggesting that E2 and NMDAR coactivation resulted in persistent increased connectivity (Fig. 5 A and B). On the contrary, acute E2 (30 min) and NMDAR activation (30 min) individually did not sustain increased spine numbers (Fig. 5B). Collectively, these data reveal that coordinated E2 and NMDAR activity results in a persistent enhancement of a form of two-step wiring plasticity.
Fig. 5.
Persistent effects of combined E2 and NMDAR activity. (A) Schematic of long-term combined E2 and NMDAR activation experiments. Neurons were treated with E2 for 0 or 30 min; for 30 min with E2 followed by activation of NMDAR for 30 min; or activation of NMDAR alone for 30 min. Cells were fixed 24 h after stimulation. (B) Effect of long-term combined E2 and NMDAR activation on dendritic morphology. Cells were treated as described in A. Treated cells were stained for bassoon. The total number of spines (color stripe bars) and the number of spines colocalizing with bassoon (gray and color stripe bars) were measured. (C) Model of two-step wiring plasticity. E2 treatment increases synaptogenesis, representing the sample step; yellow circles represent nascent connections. If a second stimulus is not applied (i.e., no hold), neuronal connectivity returns to control levels. NMDAR activation alone increases the strength of existing contacts. NMDAR activation after E2 treatment, representing the hold step, sustains and increases the strength of existing and novel connections. ***, P < 0.001. (Scale bar, 5 μm.)
Discussion
In this study we sought to investigate mechanisms underlying cortical plasticity. We report a mechanism for two-step wiring plasticity whereby E2-induced increases in synaptic connectivity are sustained by subsequent NMDAR activation. Acute E2 treatment increases spine density through a Rap-dependent pathway and generates anatomically silent synapses with depressed AMPAR transmission. Subsequent NMDAR activation, mediated via a CaMKII pathway, sustains and potentiates E2-induced nascent silent synapses. These newly formed connections persist for extended periods (24 h) (Fig. 5C). Together, these data identify physiological signals and molecular mechanisms that potentially underlie wiring plasticity that may be important for cortical plasticity and recovery from brain injury.
Combined Rap and Rac Signaling: A Possible Mechanism for Wiring Plasticity?
Use of an easily manipulated cultured cortical neuron system in this study has allowed us to elucidate the molecular components critical for rapid E2 effects. We identify that a Rap/AF-6/ERK1/2 pathway is critical for the E2-mediated increase in spine density. Reduction in Rap activity alone does not result in a decrease in spine number (Fig. S2A) (7). Therefore, it is interesting to speculate that the small decrease in Rac activity observed 60 min after E2 treatment may be critical for the transient nature of E2 actions on spines. This small inhibition in Rac activity may be sufficient to drive E2-induced spine numbers back to a level similar to that of the control, because previous studies have shown that Rac inhibition reduces spine numbers (25). Conversely, activation of Rac via an NMDAR/CaMKII pathway leads to larger spines and enrichment of synaptic GluR1 (6). Hence, NMDAR activation after E2 treatment results in stabilization of spine numbers and enhanced synaptic transmission via a CaMKII/Rac pathway; in the absence or reduction of Rac activity, these E2-induced novel thin spines retract. Together, these data are consistent with a sample-and-hold model in which novel, silent spines, induced through a Rap-dependent pathway, sample only presynaptic contacts; subsequently a Hebbian-like mechanism stabilizes and potentiates nascent spines through a Rac-dependent pathway. Although previous studies have described wiring plasticity theoretically and observed it phenomenologically in vivo (1–3), the underlying mechanisms have yet to be identified. Therefore, we suggest that a combination of Rap and Rac signaling initiated by E2 and NMDAR activation, respectively, may represent the mechanistic underpinnings of a form of wiring plasticity.
Formation of Silent Synapses.
There is much evidence for the presence of a population of synapses that contain NMDARs but lack AMPARs (21, 22), which can be potentiated to produce enhanced synaptic activity (24). Silent synapses can be formed by removing AMPARs from spines (22) or by generating novel spines that lack AMPARs but contain NMDARs (21). This model is consistent with the transient formation of silent synapses through the bidirectional trafficking of GluR1 and NR1 that we describe in this study. A correlate to the formation of silent synapses is a reduction in AMPAR transmission (22, 23). Indeed, we also see a reduction in AMPAR transmission through a transient decrease in mEPSC frequency. Although our data support a postsynaptic role of E2 through GluR1 trafficking, we cannot rule out presynaptic mechanisms contributing to these effects. Interestingly, chronic treatment with E2 (≥24 h) leads to an increase in the NMDAR/AMPAR ratios, which is suggested to underlie chronic E2-mediated LTP enhancement (16). These effects are tamoxifen-sensitive and depend on NMDAR activity (16). In contrast, we show that the formation of silent synapses by the bidirectional trafficking of glutamate receptors induced by acute E2 treatment is insensitive to tamoxifen and NMDAR blockade. Therefore, the genomic and nongenomic regulation of synaptic plasticity by E2 is mediated via distinct pathways.
Role of E2 in the Cortex.
The list of neuromodulators and neurotransmitters is constantly growing, and recent evidence has identified neurosteroids as an attractive group of molecules for this role. A substantial body of work demonstrates that the rapid actions of E2 affect cortically based cognitive function. Object recognition (13, 14), reference memory (15), and aggression (26) have been shown to be influenced by acute E2 treatment. Furthermore, long-term potentiation and long-term depression in the forebrain are both enhanced by acute E2 administration (11, 12). Collectively, these studies suggest that E2 can rapidly influence cortically mediated processing and behavior.
E2 is produced rapidly and locally in male and female brains, specifically at presynaptic terminals (8, 9). As a result of its lipophilic nature, the rapid actions of E2 would not be restricted to single synapses and could influence multisynapse connectivity. Thus, E2 is well suited to initiate such a polysynaptic form of wiring plasticity.
The identity of the receptor(s) responsible for rapid E2 effects remains elusive and may be confounded in part by the diverse effects E2 has in different brain regions. The ERα nuclear receptor has been shown to be important for E2 effects in the hippocampus (17), and a recent study has shown that spine density in hippocampal neurons increases after 1 h of E2 treatment and is sensitive to ERα and NMDAR antagonism (16). Importantly, these findings are distinct from our observations and may represent a parallel mechanism by which E2 increases spine density in a cell-type specific manner. Alternatively, other studies have implicated orphan G protein-coupled receptors in rapid steroidal responses (27), and an uncharacterized G protein-coupled receptor may mediate some of the rapid actions of E2 in the neocortex (28). In this study, we find that rapid E2 actions on cortical neurons are insensitive to the ERα nuclear receptor modulator tamoxifen and occur in the presence of NMDAR blockade, suggesting that these receptors are not involved in the rapid actions we observe.
Neural Connectivity, Behavior, and Brain Injury.
Our study describes a mechanism to increase neuronal connectivity that can be stabilized by synaptic activity. As E2 can be rapidly produced in response to sensory information, it is interesting to hypothesize that E2 acts to increase a cell's readiness to respond to activity-dependent stimuli. For example, upon entering a novel area, it would be extremely beneficial for an animal to efficiently encode information about the environment such as the presence of food or a predator (13). Therefore, the ability of E2 to transiently increase neural connectivity in distinct cortical subregions that can in turn be selectivity reinforced would allow enhanced encoding of salient information by the animal. Long-term persistence of these circuit alterations would permit them to influence subsequent behavior.
In addition to its role in information processing, this form of wiring plasticity may have important therapeutic relevance. After ischemia, an increase in spine turnover is observed (29), as is an increase in E2 synthesis (30), which is suggestive of a putative natural recovery mechanism. Animal models of stroke have demonstrated that E2 treatment after ischemic insult significantly improves cortical memory normally disrupted by ischemia (31). It is intriguing to speculate that E2-induced wiring plasticity may in part underlie this recovery and that this mechanism of increased connectivity may be exploited to aid in the recovery from cortical dysfunction induced by brain injury.
Methods
Neuronal Culture and Treatments.
Cortical neurons were treated with 10 nM E2 in ACSF with 200 μM APV for the appropriate times on DIV28 neurons. Inhibitors were added 90–30 min before treatment with E2. See SI Methods for more details.
AMPA Receptor Surface Labeling and Immunostaining.
Live cells were incubated with n-GluR1 Ab at 4°C for 30 minutes and fixed for 10 minutes in 4% formaldehyde. Cells were then processed for immunocytochemistry. See SI Methods for more details.
Electrophysiology.
AMPAR mEPSCs were measured on DIV28 neurons by whole cell patch clamp recordings with a gap free protocol using pClamp10 (Molecular Devices) and an Axopatch 200B amplifier (Axon Instruments). See SI Methods for additional methods.
Supplementary Material
Acknowledgments.
We thank G. Shepherd, D. Chklovskii, and C. Woolley for stimulating discussion and P. D. Evans and J. Radulovic for critical reading. This work was supported by the American Heart Association (D.P.S. and K.W.), Autism Speaks (D.P.S.), National Alliance for Research on Schizophrenia and Depression, National Institutes of Health/National Institutes of Mental Health Grant MH071316 (to P.P.), and National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant NS44322 (to G.T.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801581105/DCSupplemental.
References
- 1.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 2.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Le Be JV, Markram H. Spontaneous and evoked synaptic rewiring in the neonatal neocortex. Proc Natl Acad Sci USA. 2006;103:13214–13219. doi: 10.1073/pnas.0604691103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 5.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 8.Yague JG, et al. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138:389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 11.Mukai H, et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 12.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 13.Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- 14.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinopoli KJ, Floresco SB, Galea LA. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol Learn Mem. 2006;86:293–304. doi: 10.1016/j.nlm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kretz O, et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laufs U, et al. Down-regulation of Rac-1 GTPase by estrogen. J Biol Chem. 2003;278:5956–5962. doi: 10.1074/jbc.M209813200. [DOI] [PubMed] [Google Scholar]
- 21.Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci USA. 2006;103:4344–4351. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beique JC, et al. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci USA. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: Evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Trainor BC, Lin S, Finy MS, Rowland MR, Nelson RJ. Photoperiod reverses the effects of estrogens on male aggression via genomic and nongenomic pathways. Proc Natl Acad Sci USA. 2007;104:9840–9845. doi: 10.1073/pnas.0701819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava DP, et al. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toran-Allerand CD, et al. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saleh TM, Connell BJ, Legge C, Cribb AE. Estrogen synthesis in the central nucleus of the amygdala following middle cerebral artery occlusion: Role in modulating neurotransmission. Neuroscience. 2005;135:1141–1153. doi: 10.1016/j.neuroscience.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 31.Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006;49:246–260. doi: 10.1016/j.yhbeh.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.