Abstract
Placental malaria (PM) caused by Plasmodium falciparum contributes significantly to infant mortality in sub-Saharan Africa and is associated with pregnancy loss. We hypothesized that fetal genes that modify PM would be associated with fetal fitness. During PM, placental trophoblasts produce soluble fms-like tyrosine kinase 1 (sFlt1), also known as soluble VEGF receptor 1, an angiogenesis inhibitor associated with preeclampsia. Here we present a study examining the genotype of the fms-related tyrosine kinase 1 (FLT1) 3′ UTR in Tanzanian mother–infant pairs. First-time mothers suffer the most PM, and newborn FLT1 genotype distribution differed by birth order, with newborns of first-time mothers outside of Hardy–Weinberg equilibrium (HWE) during peak PM season. Among first-time but not other mothers, maternal FLT1 genotype was associated with a history of prior pregnancy loss. During PM, newborn FLT1 genotype was associated with low birth weight and placental inflammatory gene expression. FLT1 genotype was also associated with Flt1 levels among study subjects and in vitro. Thus, FLT1 variants confer fetal fitness in utero and are associated with the maternal immune response during PM. This indicates that FLT1 is under natural selection in a malaria endemic area and that human exposure to malaria can influence the evolutionary genetics of the maternal-fetal relationship.
Keywords: angiogenesis, parasitology, pregnancy, placenta, Plasmodium falciparum
In Africa, 30 million women living in malaria-endemic areas become pregnant each year and are at risk for placental malaria (PM), which is estimated to cause nearly one-third of perinatal mortality (1), including stillbirths (2). Plasmodium falciparum-infected erythrocytes adhere to chondroitin sulfate A and sequester in the maternal circulation of the placenta (3). First-time mothers have the highest rates of PM and of severe placental inflammation, which is specifically related to poor outcome. PM is less frequent and severe in multiparae, who have developed antibodies against chondroitin sulfate A-adherent infected erythrocytes (4).
Trophoblasts secrete soluble fms-like tyrosine kinase 1 (sFlt1) into the maternal circulation (5), and levels are elevated during preeclampsia (6, 7). Adenoviral delivery of sFlt1 in a rat model causes the features of preeclampsia: Hypertension, proteinuria, and glomerular endotheliosis (7). During PM in first-time mothers, sFlt1 levels are elevated and maternal inflammatory cells produce its ligand, VEGF, suggesting maternal-fetal conflict (8). sFlt1 has been postulated to be involved in maternal-fetal conflict over nutrient allocation (9), but sFlt1 also has anti-inflammatory effects through VEGF antagonism (10, 11), and therefore sFlt1 might modulate maternal inflammation during PM (8) and modify fetal outcomes.
A dinucleotide repeat polymorphism, rs3138582 (TG)n 24+, is ≈3 kb downstream of the last exon of the fms-related tyrosine kinase 1 (FLT1) gene. Polymorphism length was not associated with renal disease in one study (12), and it has not been examined further in other human conditions. RNA structure prediction indicates that the repeat forms a stem-loop [supporting information (SI) Fig. S1], suggesting a possible role in message regulation or stability. Sequence analysis also suggests that repeat length has increased within the primate lineage. We therefore investigated FLT1 repeat length in Tanzanian mother–infant pairs and its relationship with poor outcomes caused by PM.
Results
Dinucleotide Repeat Is Expressed Within the FLT1 UTR.
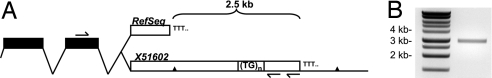
The dinucleotide repeat was included in the UTR of a FLT1 transcript (X51602) obtained from a placental cDNA library (13); however, subsequent sequences including RefSeq NM002019.3 end 2.5 kb upstream from the repeat (Fig. 1A). To confirm that the repeat is expressed within the UTR of FLT1, we designed primers targeting the last exon and the UTR downstream of the repeat. We amplified a ≈3-kb fragment from cDNA generated from total placental RNA by using a FLT1-specific reverse primer (Fig. 1B).
Fig. 1.
The dinucleotide repeat is expressed within the FLT1 UTR. (A) Diagram of the FLT1 3′ UTR, not drawn to scale. Coding exons are indicated in black and the UTR in white, showing both the RefSeq annotation and X51602, which includes the dinucleotide repeat. The locations of the two SNPs examined in this study are indicated as triangles. Primer sites for both the reverse transcriptase reaction and PCR are indicated. (B) Amplification of a ≈3-kb product using primers encompassing the last exon of the FLT1 gene and the dinucleotide repeat (expected size, 3436 bp).
FLT1 Repeat Length Is Diverse in the Tanzanian Study Population.
The study reported here included pregnant women who enrolled in the Mother–Offspring Malaria Studies Project and delivered singleton newborns in Muheza, Tanzania, an area of intense malaria transmission (14). Of mothers having their first live delivery (nulliparous), 19% had active PM at the time of delivery, compared with 6% of mothers having their third or subsequent live delivery (multiparous). DNA for genotyping was available from 239 infants and 235 mothers from nulliparous pregnancies and 359 infants and 330 mothers from multiparous pregnancies. The repeat length distribution in Tanzania was more diverse than previously reported in countries free of malaria (Fig. 2A, graph). The shortest and most frequent (24 repeats) polymorphism had a frequency of 36% compared with reported frequencies within the United States and the United Kingdom of 68% (13) and 88% (12), respectively.
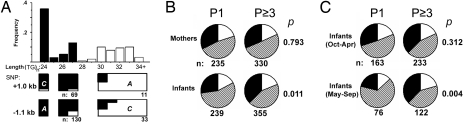
Fig. 2.
Newborn genotype at the FLT1 locus differs according to maternal parity and season of birth. (A) Frequency distribution of FLT1 3′ UTR repeat lengths, with S and L allele classification shown on graph as black and white bars, respectively. Relative proportion of flanking SNP variants, for individuals homozygous for specific repeat lengths with the reference allele (i.e., allele most common in Caucasians), is shown as black in boxes below graph. (B) Genotypes of mothers and their newborns, shown as proportions stratified by parity. (C) Genotypes of newborns according to season of birth, shown as proportions stratified by maternal parity. The proportions of SS and LL homozygotes are represented in black and white, respectively, with the SL heterozygotes in a hatched pattern. P values were calculated by using χ2 test (2 × 3) across all genotypes. P1, nulliparous pregnancy; P≥3, multiparous pregnancy.
The frequencies of dinucleotide repeat polymorphisms >27 repeats in length formed a separate normal distribution, and these were classified as the long (L) allele of the repeat polymorphism, with those 27 repeats or less classified as the short (S) allele. SNPs that flanked the dinucleotide repeat region and that varied in frequency between Caucasian and Yoruba populations were identified by using the International HapMap (15). These SNPs were sequenced for individuals in the study who were homozygous for discrete dinucleotide repeat lengths. The SNPs were linked to the S and L alleles (Fig. 2A, boxes below graph), indicating that the S and L alleles form discrete haplotypes. SNPs associated with Caucasian ethnicity were linked to S alleles and those associated with African ancestry to L alleles. The frequency of the S allele did not differ by maternal affiliation with the major tribes of Bondei and Sambaa or the >50 minor tribes (n = 457, χ2 P = 0.40) living in our study area under endemic malaria transmission. The genotypes of all subjects were within Hardy–Weinberg equilibrium (HWE) (n = 1159, P = 0.99).
Infant FLT1 Genotype Differs by Parity and Birth Season.
PM is most frequent and most severe in first-time mothers; therefore infant and maternal genotypes were stratified by maternal parity (Fig. 2B). Overall, the frequency of the S allele was similar between infants and mothers. However, genotype distribution among infants, but not mothers, differed by parity. Fewer S-homozygous (SS) offspring were born to first-time mothers than to multiparous mothers. Although not significant, there was a trend toward increased numbers of SS offspring born to multiparous mothers. Allele frequencies are reported in Table S1. PM had a seasonal variation during this study that peaked during May–September (with PM in 28% of first-time mothers) and troughed during October–April (with PM in 15% of first-time mothers). During the peak PM season, significantly fewer SS infants were born to first-time mothers than to multiparous mothers (Fig. 2C). Infants born to first-time mothers during peak PM season were outside of HWE (P = 0.018), consistent with disproportionate mortality for SS fetuses. All other groups were within HWE.
FLT1 Genotype Is Associated with History of Pregnancy Loss and Low Birth Weight.
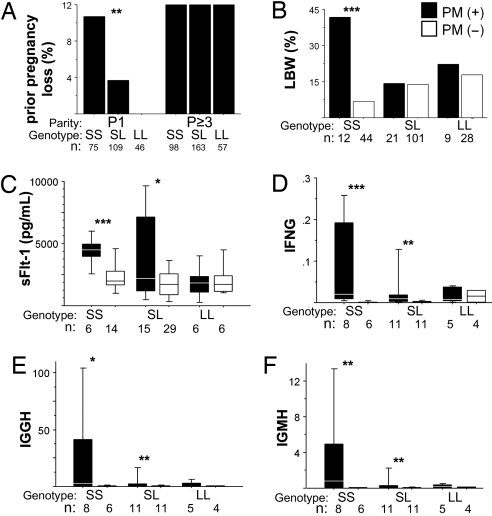
The proportion of mothers who reported prior pregnancy loss varied by parity and FLT1 genotype (Fig. 3A). Among mothers delivering their first live infant in our study, SS mothers reported the highest rate of a prior pregnancy loss, whereas none of 46 L-homozygous (LL) mothers reported a pregnancy loss, and heterozygous (SL) mothers had an intermediate rate of loss. Thus, rates of prior pregnancy loss among nulliparae were consistent with the likelihood of having an SS fetus. Reports of multiparous mothers reflected a cumulative increase in prior pregnancy losses, with all genotypes reporting a similar rate of loss. The data suggest that mothers experience excess loss of LL offspring after their first pregnancy; however, data regarding pregnancy order of the prior losses were not available.
Fig. 3.
Association of FLT1 genotype with pregnancy outcome. (A) Rates of reported prior pregnancy loss of women stratified by parity and maternal genotype. P1, nulliparous pregnancy; P≥3, multiparous pregnancy. (B) Rates of LBW delivery for first-time mothers stratified by newborn genotype and PM status. (C) Maternal peripheral plasma sFlt1 levels of first-time mothers stratified by newborn genotype and PM status. (D–F) Placental transcript levels of inflammatory genes in first-time mothers stratified by newborn genotype and PM status. Transcript levels are shown as fold change relative to cytokeratin 7. P values calculated by χ2 test for categoric variables and t test for log-transformed sFlt1 levels and corrected cycle threshold (CT) values. *, P < 0.08; *, P < 0.05; ***, P < 0.01.
Low birth weight (LBW) is a complication of PM frequently observed in first-time mothers and predicts increased infant mortality. Rates of LBW were elevated in SS offspring, but not SL or LL offspring, born to first-time mothers with PM (Fig. 3B). In contrast, SS offspring of PM-negative first-time mothers had the lowest rates of LBW among all groups of children. Maternal genotype was not associated with LBW during PM. Rates of PM did not differ by maternal or infant genotype (data not shown), consistent with the role of maternal acquired immunity as the major determinant of PM risk. Rates of hypertension were not associated with maternal or infant FLT1 genotype.
Infant FLT1 Genotype Is Associated with Placental Inflammation.
Inflammatory responses are associated with pregnancy loss, and animal studies indicate that immune-mediated pregnancy failure is a consequence of immune activation at the maternal-fetal interface (reviewed in ref. 16). We earlier observed that during PM, sFlt1 was specifically elevated in mothers who had placental inflammation by histology (8). sFlt1 levels in maternal plasma were elevated during PM in first-time mothers of SS offspring, but not LL offspring (Fig. 3C), and were heterogenous in mothers of SL offspring. Placental transcript levels of IFN-γ and IgG and −M heavy chains were previously associated with poor pregnancy outcome in this cohort (17) and were elevated in the placentas of SS and SL but not LL infants during PM (Fig. 3 D–F). The placentas of SS infants had the greatest levels of these transcripts. sFLT1 and IFN-γ placental transcript levels approached significant correlation (R = 0.392, n = 24, P = 0.0584).
FLT1 Genotype Is Associated with Expression Levels In Vitro.
To determine whether dinucleotide repeat length may be a functional polymorphism, we examined the effect of LPS on FLT1 expression in peripheral blood mononuclear cells (PBMC) obtained from cord blood from infants of different genotypes. LPS stimulation of PBMC from SS and SL infants resulted in increased FLT1 mRNA and protein expression, whereas PBMC from LL individuals did not increase FLT1 expression (Fig. 4). PBMC from SS and SL individuals also had higher levels of FLT1 expression compared with LL individuals.
Fig. 4.
Association of FLT1 genotype with expression levels in vitro. (A) Transcript levels of the transmembrane isoform of FLT1 and (B) protein levels of Flt1 in supernatants of cultured PBMC stratified by genotype and LPS treatment (100 ng/ml for 24 h). P values calculated by paired t test for log-transformed Flt1 levels and corrected CT values. *, P < 0.05; **, P < 0.01.
Discussion
PM is a major public health problem in tropical countries, and first-time mothers are particularly affected by poor outcomes. Our data suggest that FLT1 genotype is causally related to pregnancy outcome during PM. This may be a direct effect of 3′ UTR dinucleotide repeat length, or alternatively, length may be linked to a distinct causative site. We observed alleles of 24–34 repeats in this study. Data indicate that the chimpanzee allele contains 20 repeats, whereas the rhesus and mouse contain 10–12 repeats, suggesting recent expansion in the human lineage. The dinucleotide repeat is predicted to form a stem-loop, thus repeat length may alter RNA secondary structure (Fig. S1) and regulate mRNA stability, splicing, or translation. Subsequent mRNA processing may affect the regulation of Flt1 production or downstream Flt1 signaling in the fetoplacental unit.
Functional data using PBMC suggest that the polymorphism has an effect on FLT1 mRNA and protein expression in response to LPS stimulation. A major caveat is that during pregnancy and during PM, the trophoblast is the major source of the soluble isoform of FLT1, and the trophoblast possesses unique FLT1 regulatory mechanisms (18). The effect of this polymorphism on trophoblast FLT1 expression remains to be determined.
Poor outcomes during PM are associated with placental inflammation, and the infiltrating immune cells are of maternal origin. We observed that infant FLT1 genotype was associated with pregnancy outcome during PM, and therefore we postulate that infant FLT1 genotype may modulate the maternal inflammatory response to PM. Two observations support this model: First, infant FLT1 genotype was associated with inflammatory gene expression in this study, and second, placental sFlt1 levels were previously associated with inflammation during PM (8). These relationships are intriguing because sFlt1 has anti-inflammatory effects in experimental models (10, 11). Further evidence of causation between FLT1 genotype, maternal inflammation, and sFlt1 expression during PM will require longitudinal data during PM episodes or an experimental model of PM.
Our data from Tanzania suggest that maternal malaria exerts selective pressure in utero at the FLT1 locus through pregnancy loss. SS offspring seem to be at a selective disadvantage in first-time mothers in malaria endemic areas. The data may also suggest that SS offspring are at an advantage in the absence of malaria pressure: SS offspring were slightly enriched among malaria-immune multiparous mothers, and LBW was least frequent among SS offspring born to PM-negative mothers, although neither trend attained significance. The effect of this polymorphism on pregnancy outcomes in nonmalarious areas has not been characterized, although populations in the United States and United Kingdom have a large preponderance of the SS genotype. The natural role of sFlt1 in pregnancy is not known; however, it may contribute to the pathogenesis of preeclampsia. Preeclampsia, like PM, is most frequent in first-time mothers, and sFlt1 levels are elevated in healthy first vs. second pregnancies (19). We speculate that exposure to malaria during human evolution influenced sFlt1 regulatory mechanisms, and this might continue to affect pregnancies outside of malaria endemic areas.
This study reports a human gene that confers resistance to infectious disease in utero. Because this was a community-based study with a high rate of disease, we were able to observe natural selection occurring in a human population. During a major malaria epidemic in Sri Lanka during 1934–1935, PM caused perinatal death in 67% of cases with half occurring in utero (20). Therefore epidemic PM, which largely affects nonimmune populations, may lead to large-scale selective sweeps, whereas endemic PM may influence the genotype of only first-born offspring whose mothers lack immunity to chondroitin sulfate A-adherent infected erythrocytes. These data suggest that fetal genes that modify maternal inflammation may be under natural selection secondary to malaria, thereby contributing to the evolution of the human maternal-fetal relationship.
Methods
Human Subjects.
The study reported here included nulliparae and multiparae who enrolled in the Mother–Offspring Malaria Studies Project, delivering at the Muheza Designated District Hospital between September 2002 and April 2005. Cord blood for PBMC isolation was collected from deliveries occurring at the Morogoro Regional Hospital during 2007. Women provided informed consent for themselves and their offspring. Birth weight was measured on digital scales and LBW defined as ≤2,500 g. PM was diagnosed by microscopic examination of placental blood obtained by mechanical extraction from the placenta after delivery.
FLT UTR Amplification.
cDNA was generated from placental RNA and isolated as described in a following section by using SuperScript III (Invitrogen) and a FLT1-specific reverse primer TGCCACAGGATGTTTTAACG. PCR was performed by using ex-Taq polymerase (TaKaRa) and the primers CTTCACCTGGACTGACAGCA (forward) and GGTTCGAAAACCCCATACAA (reverse) with an annealing temperature of 59°C for an expected product size of 3,436 bp.
FLT1 Genotyping.
DNA was extracted from filter–paper blood spots and frozen blood pellets (Qiagen). The FLT1 3′ UTR dinucleotide repeat rs3138582 was PCR amplified by using AmpliTaq gold (ABI) and the primers TGGCCGACAGTGGTGTAAC (forward) and AACTTTAAAATTCCAGTTTCCTTAAA (reverse) with 5′ 6-FAM modification of the forward primer and an annealing temperature of 50°C. Fragment length was determined by capillary electrophoresis (ABI). The following SNPs were PCR amplified: rs9554314 (C/A) at −1.14 kb and rs17086497 (A/C) at +0.99 kb from the dinucleotide repeat. The following primer pairs were used: AGCAATCCACTGTTGCCTCT (forward) with GGGAGACAGGGTAGGAAAGG (reverse) and TTTCCAGAGCCATGAGAACA (forward) with GGCAAGAGGCATTTTGTCTT (reverse) for each SNP, respectively. PCR products were purified and sequenced by using the reverse primers for each SNP.
Flt1 ELISA.
sFlt1 levels in maternal plasma were assayed as previously described (8). Briefly, peripheral blood was collected in citrate phosphate dextrose immediately after delivery, and plasma was separated and frozen at −80°C. Soluble Flt1 levels in peripheral plasma were determined in duplicate by ELISA kit DVR100A (R&D Systems). Cell culture media was centrifuged and Flt1 levels determined in duplicate by ELISA kit DVR100B (R&D Systems).
Quantitative PCR.
Placentas were collected at delivery, and fresh tissue was frozen in liquid nitrogen. Total RNA was extracted from cryosections as previously described (17) or harvested from PBMC samples by using RNeasy Mini Kits (Qiagen). cDNA was synthesized by using a SuperScript III enzyme (Invitrogen Life Technologies) and anchored oligo(dT)20 primers. Real-time PCR was performed in duplicate by using SYBR Green Master Mix and an ABI Prism 7500 system (Applied Biosystems). Quantitative PCR was performed by using previously specified primers (17) in addition to the transmembrane isoform of FLT1: AGGGGAAGAAATCCTCCAGA (forward) and GAGGTTTCGCAGGAGGTATG (reverse) and GAPDH: ACTTCAACAGCGACACCCACTC (forward) and CACCCTGTTGCTGTAGCCAAA (reverse). Threshold cycles (CT) were calculated and normalized to the CT of KRT7 or GAPDH for placental or PBMC samples, respectively.
Cell Culture.
Cord blood samples were obtained immediately after delivery. PBMC were isolated by using lymphocyte separation media, frozen in RPMI with 40% FCS and 10% DMSO, stored in liquid nitrogen, and shipped to Seattle on dry ice. FLT1 genotype was determined as previously described. Cell viability was determined by trypan blue exclusion, and cells were plated at 1 × 106/ml. Cells were cultured in RPMI-1640 (Sigma) supplemented with 10% heat-inactivated FCS, penicillin, streptomycin, and gentamicin. After 24 h of incubation, 100 ng/ml LPS from Escherichia coli O55:B5 (Sigma) was added for another 24 h.
Statistical Analysis.
Analyses were performed by using Statview (SAS). P values were calculated by χ2 test for categorical variables, and t test for log-transformed Flt1 levels and corrected CT values. For cell culture assays, paired t test was used. Regression coefficients were calculated by using simple regression analysis. A P value of <0.05 was considered significant.
Supplementary Material
Acknowledgments.
This article is dedicated to the memory of Prof. Robert Desowitz, the eminent parasitologist and popular science author, whose discussions on prenatal immune priming contributed to our studies of pregnancy malaria and its effects on offspring. Project nurses processed the samples used in these studies, and project technicians interpreted the blood smears. Ellen Sisk (Seattle Biomedical Research Institute) contributed to genotyping and fragment analysis. Cate Speake (Seattle Biomedical Research Institute) contributed to FLT1 PCR analysis. This work was supported by funds from the Bill and Melinda Gates Foundation and National Institutes of Health Grant R01AI52059 (to P.E.D.). A.M. received support from an American Heart Association predoctoral fellowship and a Poncin scholarship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 14243.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803657105/DCSupplemental.
References
- 1.Garner P, Gulmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev. 2006;4:CD000169. doi: 10.1002/14651858.CD000169.pub2. [DOI] [PubMed] [Google Scholar]
- 2.van Geertruyden JP, Thomas F, Erhart A, D'Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg. 2004;71:35–40. [PubMed] [Google Scholar]
- 3.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 4.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 5.Clark DE, et al. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 6.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Med. 2006;3:e446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan HT, Haig D, Karumanchi SA. Angiogenic factors in the pathogenesis of preeclampsia. Curr Top Dev Biol. 2005;71:297–312. doi: 10.1016/S0070-2153(05)71009-7. [DOI] [PubMed] [Google Scholar]
- 10.Miotla J, Maciewicz R, Kendrew J, Feldmann M, Paleolog E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab Invest. 2000;80:1195–1205. doi: 10.1038/labinvest.3780127. [DOI] [PubMed] [Google Scholar]
- 11.Yano K, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203:1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parry RG, Gillespie KM, Clark AG, Mathieson PW. Dinucleotide repeat polymorphisms within the Flt-1 gene in minimal change nephropathy. Eur J Immunogenet. 1999;26:321–323. doi: 10.1046/j.1365-2370.1999.00161.x. [DOI] [PubMed] [Google Scholar]
- 13.Polymeropoulos MH, Rath DS, Xiao H, Merril CR. Dinucleotide repeat polymorphism at the human fms-related tyrosine kinase gene (FLT1) Nucleic Acids Res. 1991;19:2803. doi: 10.1093/nar/19.10.2803-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutabingwa TK, et al. Maternal malaria and gravidity interact to modify infant susceptibility to malaria. PLoS Med. 2005;2:e407. doi: 10.1371/journal.pmed.0020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a th2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 17.Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol. 2007;179:557–565. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- 18.Nagamatsu T, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–48345. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 19.Wolf M, et al. Circulating levels of the antiangiogenic marker sFlt-1 are increased in first versus second pregnancies. Am J Obstet Gynecol. 2005;193:16–22. doi: 10.1016/j.ajog.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Wickramasuriya GA. Malaria and Ankylostomiasis in the Pregnant Woman. London: Oxford Univ Press; 1936. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.