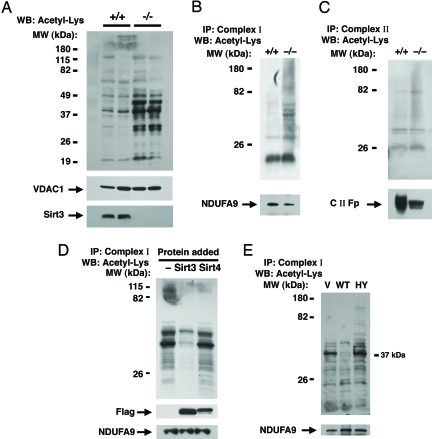
Fig. 2.
Sirt3 regulates mitochondrial protein acetylation. (A) Western blot (WB) analysis for internal acetyl-lysine residues using total liver mitochondrial protein extracts from two age-matched wild-type (+/+) or Sirt3−/− mice. The mitochondrial protein VDAC1 is used as a loading control, and Sirt3 expression is also shown. (B) Levels of acetylation from immunocaptured hepatic Complex I in a wild-type versus Sirt3−/− mouse. Subunit 9 (NDUFA9) of Complex I was used as a loading control for the immunocapture. (C) Similar acetylation analysis for immunocaptured Complex II. The 70-kDa Fp subunit of Complex II (C II-Fp) was used as a loading control. (D) Sirt3 deacetylates Complex I in vitro. Complex I was isolated from nicotinamide-treated HeLa cells and incubated for 2 hours in vitro with deacteylase reaction buffer only (−) or with reaction buffer containing purified Sirt3 or Sirt4. The level of the Complex I protein NDUFA9 is shown as a loading control as is the level of exogenously added Flag-tagged Sirt3 and Sirt4. (E) Level of in vivo Complex I acetylation in HeLa cells transfected with empty vector (V), wild-type Sirt3 (WT), or a deacetylase inactive form of Sirt3 (HY). IP, immunoprecipitation; MW, molecular mass.