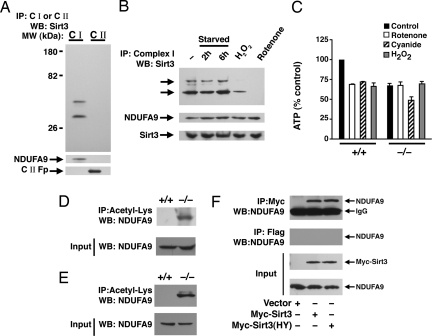
Fig. 3.
Sirt3 associates with Complex I of the ETC. (A) Sirt3 associates with Complex I. Equal amounts of HeLa cell lysate was used to immunocapture either Complex I or Complex II. These ETC components were then resolved on SDS/PAGE and probed for association with Sirt3. Both the short and long form of human Sirt3 associates with Complex I. The purity of the immunocapture complexes are demonstrated by probing the stripped blot for the Complex I component NDUFA9 and the 70-kDa Fp subunit of Complex II. (B) Reversible association of endogenous Sirt3 with Complex I. Immunocaptured Complex I was probed for associated Sirt3 under fed conditions (−), after 2 or 6 h of starvation, after hydrogen peroxide (0.5 mM, 30 min) treatment, or after rotenone (10 μM, 30 min) treatment. The arrows indicate the short and long form of human Sirt3. Below, total levels of Sirt3 or the Complex I component NDUFA9 were assessed for each condition by using 30 μg of mitochondrial protein lysate. (C) ATP levels in wild-type (+/+) or Sirt3−/− MEFs under basal conditions (black bars), or after a 30-min exposure to rotenone (50 μM; white bars), cyanide (20 μM; hatched bars), or hydrogen peroxide (0.5 mM; gray bars). Basal levels of ATP are reduced in the Sirt3−/− cells and are relatively resistant to rotenone or hydrogen peroxide challenge but exhibit normal ATP sensitivity to cyanide. (D and E) Levels of acetylation of NDUFA9 in MEFs (D) or liver protein lysates from wild type (+/+) or Sirt3−/− mice (E). (F) HeLa cells were transfected with a myc-tagged empty vector, wild-type Sirt3, or a deacetylase-inactive Sirt3(HY) and equal amounts of lysate immunoprecipitated with a myc-epitope antibody or an irrelevant Flag-epitope antibody. Sirt3 immunoprecipitation reveals the presence of coprecipitated endogenous NDUFA9. IP, immunoprecipitation; WB, Western blotting.