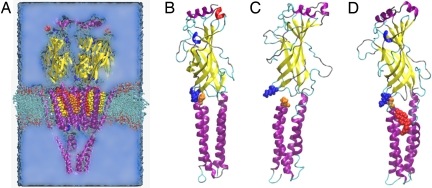
Fig. 4.
Mechanical decoupling of TM and agonist-binding domains in system without cholesterol. (A) All simulations contained the nAChR structure as described in 2BG9 (cartoon, colored by structure: α-helix is purple, β-sheet is yellow, turn is cyan, coil is silver, 310-helix is blue, π-helix is red), water (blue medium), ions (not shown), phospholipid (licorice), and 0–15 cholesterol molecules (space-filling, colored as in Fig. 1). (B–D) Agonist-binding and TM domain of γ subunit are shown at start of all simulations (B), at the end of control simulation without cholesterol (C), and at the end of simulation with cholesterol bound to sites A, B, and C (D). Shown in space-filling representation are K45 (blue), P280 (orange), and cholesterol in binding site C (red). The K45–P280 contact anchors the agonist-binding domain to the TM domain. When it is broken, as at the end of the control simulation shown in C, the agonist-binding domain tilts away from its starting orientation and the TM domain, so that the only communication between the two domains is through the flexible peptide chain linking M1 to the agonist-binding domain. Furthermore, β-sheets in the agonist-binding domain begin to unravel, reducing mechanical cohesion within the agonist-binding domain. Breaking of the K45–P280 contact is caused by the collapse of the loop containing P280 into the cholesterol-binding sites; occupation of these sites by cholesterol (D) precludes such a collapse.