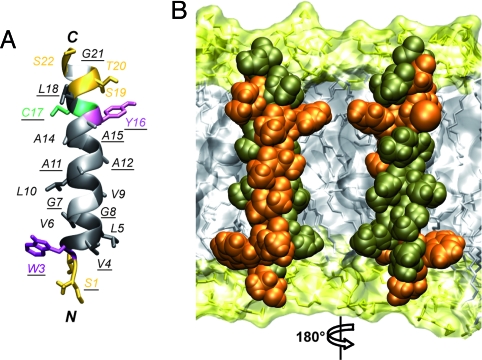
Fig. 2.
Structure of the NS4A transmembrane segment. (A) Ribbon representation of the best representative structure of NS4A[1-22]* selected from the final set of 26 calculated NMR structures (BMRB entry 15580). Residue side chains are shown as sticks and are colored on the basis of the chemical properties of their side chains (hydrophobic, dark gray; polar, yellow). Gly are light gray. Tyr, Trp, and Cys are violet, magenta, and green, respectively. Fully conserved residues are underlined. (B) Amino acid van der Waals representation and tentative position of NS4A amino acids 1–22 within a phospholipid bilayer. Fully and less conserved residues are colored orange and bronze, respectively. Orientation of the left structure is the same as in image A. Note that the majority of fully conserved residues are located on one side of the transmembrane α-helix. The membrane is represented as a simulated model of a 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayer (http://moose.bio.ucalgary.ca). Polar heads and hydrophobic tails of phospholipids (surface and stick structures) are light yellow and gray, respectively.