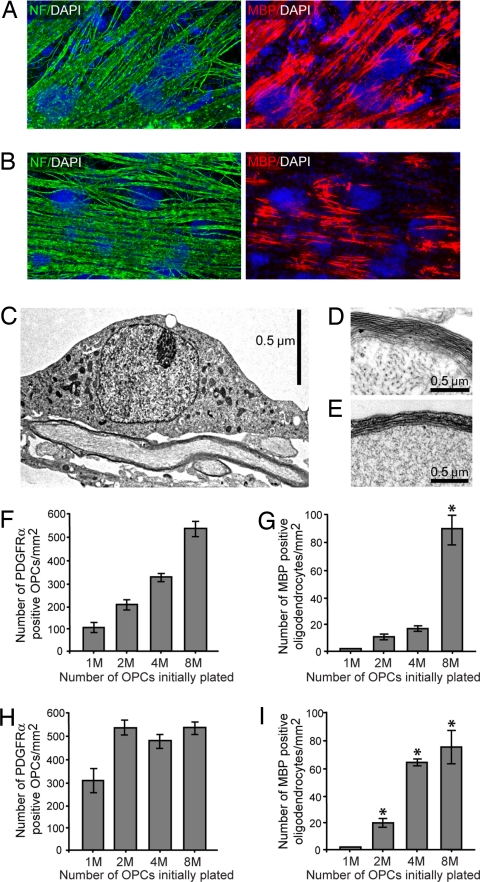
Fig. 2.
The induction of differentiation at a critical density of OPCs does not require dynamic axonal signaling. (A and B) Immunostaining of cocultures five days after seeding a high density of OPCs. Axons (green) are identified by immunostaining for NF. (A) Live axons. (B) Axons fixed with 4% paraformaldehyde to eliminate dynamic axonal signaling. Immunostaining with MBP (red) demonstrates that fixed axons support oligodendrocyte differentiation and myelination in a manner comparable to live axons. Nuclei are stained by using DAPI (blue). (C–E) Electron micrographs of oligodendrocyte myelination. The compact, multilayered myelin formed by oligodendrocytes on live axons (C and D) is also seen in fixed axon cocultures (E). (F–I) Quantification of the critical density of OPCs required to induce population-wide differentiation after five days in culture. A density of ≈500–600 PDGFRα+ OPCs on axons per millimeter squared is required for the induction of oligodendrocyte differentiation on both fixed axons (F and G) and on live axons (H and I). Here, we define population-wide differentiation as ≈60–100 MBP+ oligodendrocytes per millimeter squared. Note that because OPCs seeded onto fixed axons (F and G) fail to proliferate, population-wide differentiation is induced only when 8 million (8M) OPCs are initially plated. In contrast, OPCs seeded onto live axons (H and I) at an initial density of 2M, 4M, or 8M cells will all reach the critical density required for differentiation. Note that after five days, OPCs seeded onto live axons at an initial density of 2M have just reached the critical density and are just beginning to differentiate. Error bars represent standard deviation. MBP+ oligodendrocytes were quantified by counting 20 fields/coverslip, 3 coverslips/density. P < 0.01 versus 1M density cultures (Student–Newman–Keuls post hoc comparison after one-way ANOVA).