Figure 1.—
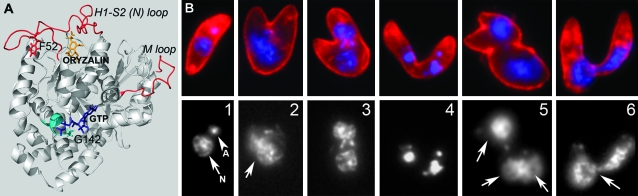
(A) The F52Y and G142S α-tubulin point mutations confer 13 and 1 μm oryzalin resistance when integrated into wild-type (sensitive) T. gondii parasites. The F52Y mutation (red) is located in the H1-S2 (N) loop (red) of α1-tubulin. The computationally determined dinitroaniline-binding site is below this loop (oryzalin in orange). Interactions between the H1-S2 (N) loop and the M loop of adjacent dimers coordinate lateral associations between protofilaments in the microtubule lattice. The G142S mutation is located in the core of α1-tubulin and the G142 residue represents the initial glycine in the GGGTGSG motif (teal), which is characteristic of tubulins. This motif is part of the GTP-binding site, which is essential for correct folding of the tubulin dimer and interacts with the phosphate portion of GTP (purple). (B) (Top) Immunofluorescence images of extracellular parasites of the G142S line labeled with DG52 (red labels the parasite surface) and DAPI (blue labels the parasite DNA). (Bottom) DNA distribution only. (1) A normal-appearing parasite has a crescent shape and DNA staining of the nucleus (arrow) and apicoplast DNA (arrowhead). (2–6) Parasite “monsters” result from replication defects due to improper coordination of spindle microtubule (nuclear division) and subpellicular microtubule (cytokinesis) functions. Although the extracellular parasites in 3 and 4 are similar to intracellular replicating stages (see diagram in Ma et al. 2007), parasites cannot complete division outside of host cells and their abnormal shape prevents invasion of new host cells. In addition, 2, 5, and 6 illustrate parasites with diffuse (2), improperly segregated (three nuclei) (5), and nonsegregated (6) nuclear staining (compare to 1).