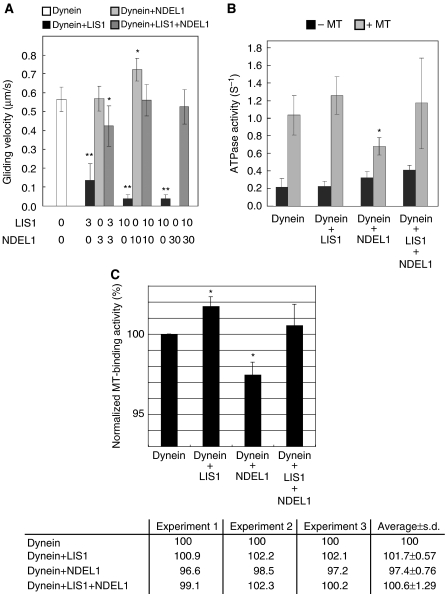
Figure 1.
Effects of LIS1/NDEL1 on the in vitro motor properties of cytoplasmic dynein. (A) Dependence of gliding velocity of microtubules (MTs) on the concentration of LIS1 and NDEL1. Molecular ratio is indicated at the bottom. Note: LIS1 displayed dose-dependent inhibition of dynein motility, whereas NDEL1 facilitated dissociation of dynein with MTs. The presence of LIS1 and NDEL1 restored dynein binding with MTs. No translocation was counted due to complete dissociation of dynein at the highest concentration of NDEL1. The P-value was calculated using a Student's t-test (*P<0.05, **P<0.01). (B) MgATPase activities of cytoplasmic dynein. Each protein was added at a 10-fold stoichiometric amount to the cytoplasmic dynein heavy chain. Left bars: activity without MTs. Right bars: calculated kcat for each protein combination. The P-value was calculated using a Student's t-test (*P<0.05). (C) MT-binding assay. The amount of cytoplasmic dynein bound to MTs was indicated in percentage (and s.d.) of total cytoplasmic dynein. LIS1 slightly increased cytoplasmic dynein binding to MTs, whereas NDEL1 reduced the cytoplasmic dynein binding. When both LIS1 and NDEL1 were present, the reduced binding is restored to the level of MTs with LIS1 alone. Three independent experiments were performed, and we found significant difference. Intensity of the band of SDS–PAGE was measured and was normalized as shown at the bottom. The P-value was calculated using a Student's t-test (*P<0.05).