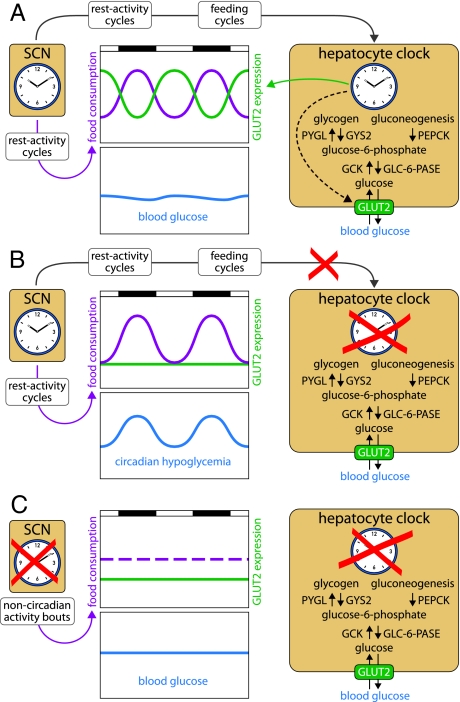
Fig. 1.
Hepatocyte oscillators drive the cyclic expression of many enzymes associated with glucose homeostasis, such as glycogen synthase (GYS2), glycogen phosphorylase (PYGL), phosphoenolpyruvate carboxykinase (PEPCK), glucokinase (GCK), glucose-6-phosphatase (GLC-6-Pase), and the glucose transporter GLUT2. (A) In wild-type animals, the SCN drives feeding rhythms and, thus, rhythms of glucose supply from nutrients. These cycles are counteracted by antiphasic rhythms of hepatic glucose export. (B) In L-Bmal1−/− mice, the floxed Bmal1 alleles have been disrupted only in hepatocytes. Because the SCN master clock is intact in these animals, their activity and feeding rhythms are normal. However, because the disabled liver clock can no longer compensate the lack of food-derived glucose by a burst of GLUT2 expression during the fasting phase, L-Bmal1−/− mice display a circadian hypoglycemia. (C) In Bmal1−/− mice, the circadian clocks are disabled in all tissues. These arrhythmic animals eat throughout the day, and the nutrient-derived glucose supply is nearly invariable over time. Although GLUT2 is expressed at low levels, it is sufficient to keep blood glucose at nearly normal (and invariable) concentrations.