Abstract
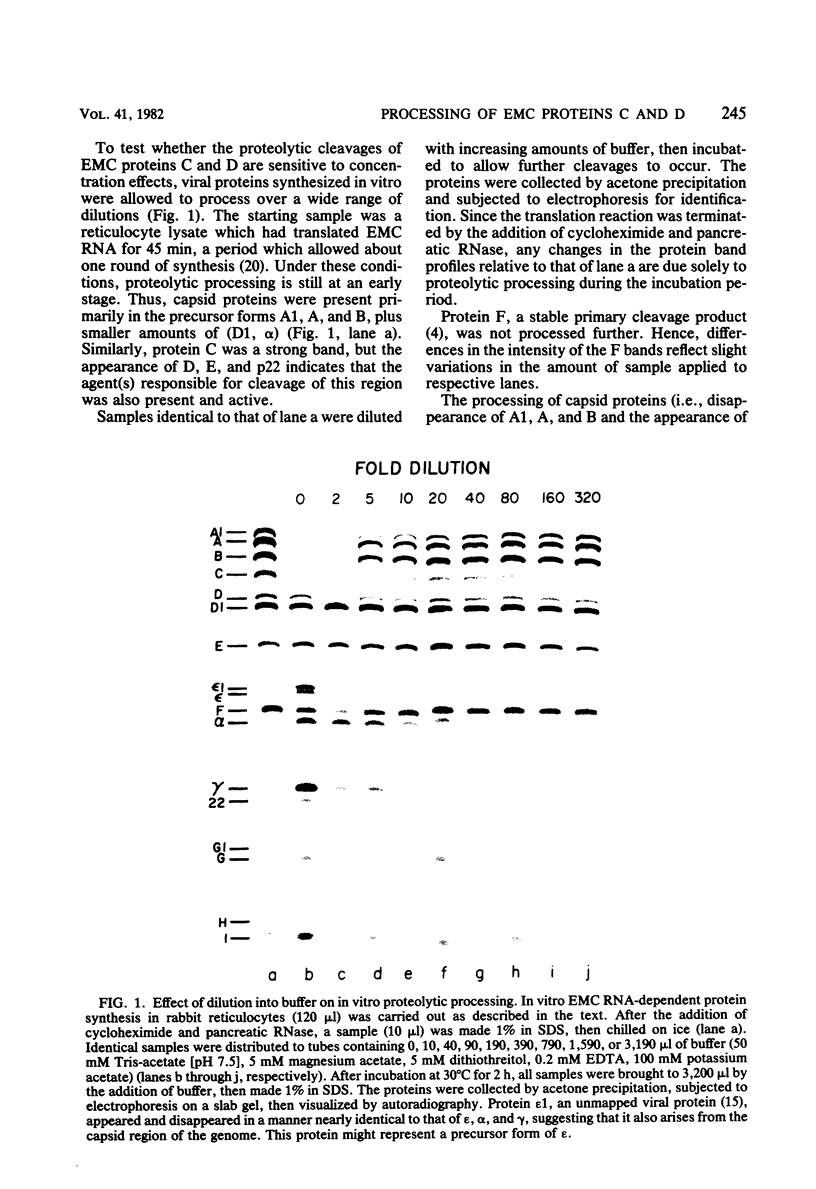
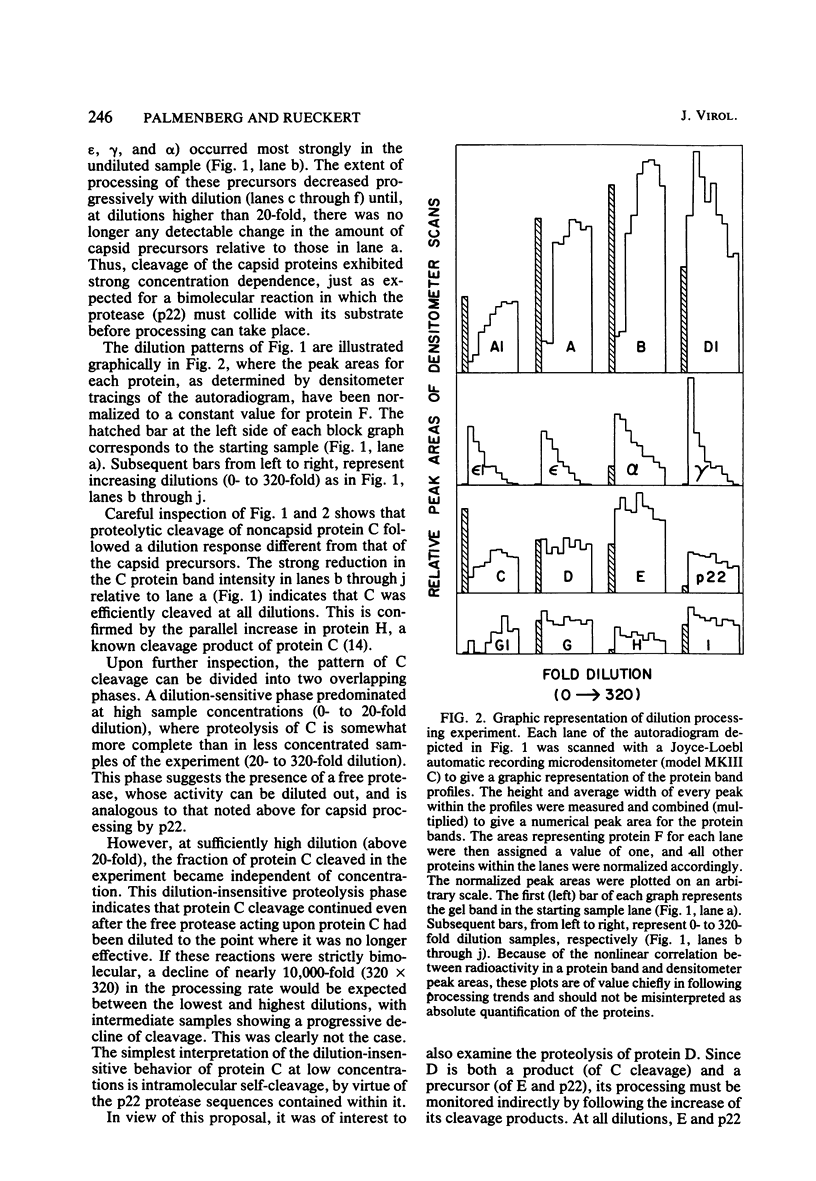
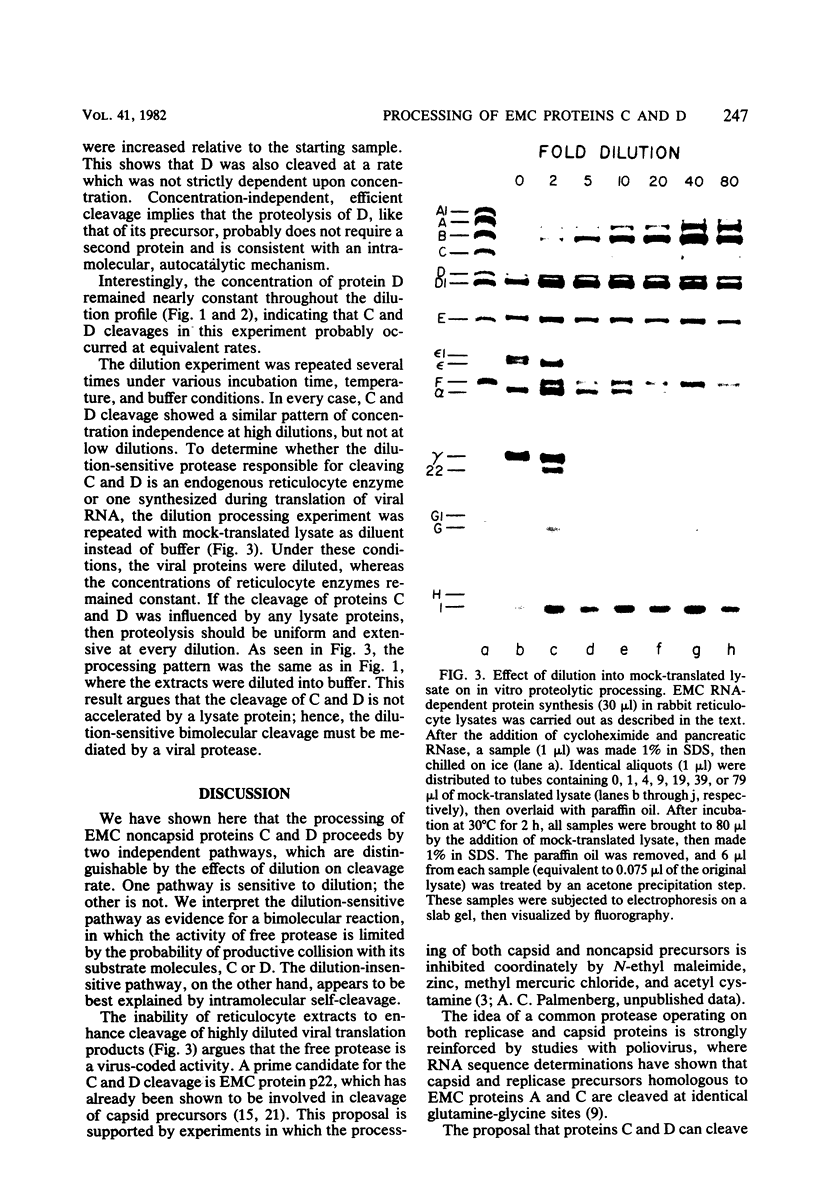
It has previously been shown that when encephalomyocarditis viral RNA is translated in cell-free extracts of rabbit reticulocytes, it synthesizes a virus-coded protease, p22, which is derived by cleavage of a precursor protein, C. Protein C is shown here to be cleaved by two different mechanisms, which were distinguished by their sensitivity to dilution. One mechanism was sensitive to dilution; the other was not. The biphasic cleavage behavior was unchanged by diluting incubation mixtures with untranslated reticulocyte extract instead of buffer, suggesting that both types of cleavage were mediated by virus translation products. It is proposed that the dilution-sensitive cleavage of protein C is due to a virus-coded protease, probably p22 itself, and that the dilution-independent cleavage is due to intramolecular self-cleavage of protein C.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustin M., Conway-Jacobs A. Intramolecular activation of porcine pepsinogen. J Biol Chem. 1971 Feb 10;246(3):615–620. [PubMed] [Google Scholar]
- Butterworth B. E., Korant B. D. Characterization of the large picornaviral polypeptides produced in the presence of zinc ion. J Virol. 1974 Aug;14(2):282–291. doi: 10.1128/jvi.14.2.282-291.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin YuV, Agol V. I. Proteolytic activity of the nonstructural polypeptide p22 of encephalomyocarditis virus. Biochem Biophys Res Commun. 1981 Feb 27;98(4):952–960. doi: 10.1016/0006-291x(81)91203-1. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin Y. V., Kazachkov Y. A., Agol V. I. Encephalomyocarditis virus-specific polypeptide p22 is involved in the processing of the viral precursor polypeptides. FEBS Lett. 1979 Dec 1;108(1):1–5. doi: 10.1016/0014-5793(79)81164-3. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Lund G. A., Scraba D. G. The isolation of Mengo virus stable non-capsid polypeptides from infected L cells and preliminary characterization of an RNA polymerase activity associated with polypeptide E. J Gen Virol. 1979 Aug;44(2):391–403. doi: 10.1099/0022-1317-44-2-391. [DOI] [PubMed] [Google Scholar]
- Marciniszyn J., Jr, Huang J. S., Hartsuck J. A., Tang J. Mechanism of intramolecular activation of pepsinogen. Evidence for an intermediate delta and the involvement of the active site of pepsin in the intramolecular activation of pepsinogen. J Biol Chem. 1976 Nov 25;251(22):7095–7102. [PubMed] [Google Scholar]
- McPhie P. A spectrophotometric investigation of the pepsinogen-pepsin conversion. J Biol Chem. 1972 Jul 10;247(13):4277–4281. [PubMed] [Google Scholar]
- Newman J. F., Cartwright B., Doel T. R., Brown F. Purification and identification of the RNA-dependent RNA polymerase of foot-and-mouth disease virus. J Gen Virol. 1979 Nov;45(2):497–507. doi: 10.1099/0022-1317-45-2-497. [DOI] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Palmenberg A. C., Golini F., Wimmer E., Rueckert R. R. Picornaviral VPg sequences are contained in the replicase precursor. J Virol. 1980 Aug;35(2):414–419. doi: 10.1128/jvi.35.2.414-419.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Kew O., Pallansch M., Rueckert R., Kaesberg P. Cell-free synthesis and processing of the proteins of poliovirus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5807–5811. doi: 10.1073/pnas.75.12.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y. V., Gorbalenya A. E., Kazachkov Y. A., Agol V. I. Encephalomyocarditis virus-specific polypeptide p22 possessing a proteolytic activity: preliminary mapping on the viral genome. FEBS Lett. 1979 Dec 1;108(1):6–9. doi: 10.1016/0014-5793(79)81165-5. [DOI] [PubMed] [Google Scholar]
- Traub A., Duskin B., Rosenberg H., Kalmar E. Isolation and properties of the replicase of encephalomyocarditis virus. J Virol. 1976 May;18(2):375–382. doi: 10.1128/jvi.18.2.375-382.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. A., Flanegan J. B. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980 Sep;35(3):732–740. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. Proteolytic activity of nerve growth factor: a case of autocatalytic activation. Biochemistry. 1979 Jul 10;18(14):3050–3055. doi: 10.1021/bi00581a022. [DOI] [PubMed] [Google Scholar]
- al-Janabi J., Hartsuck J. A., Tang J. Kinetics and mechanism of pepsinogen activation. J Biol Chem. 1972 Jul 25;247(14):4628–4632. [PubMed] [Google Scholar]