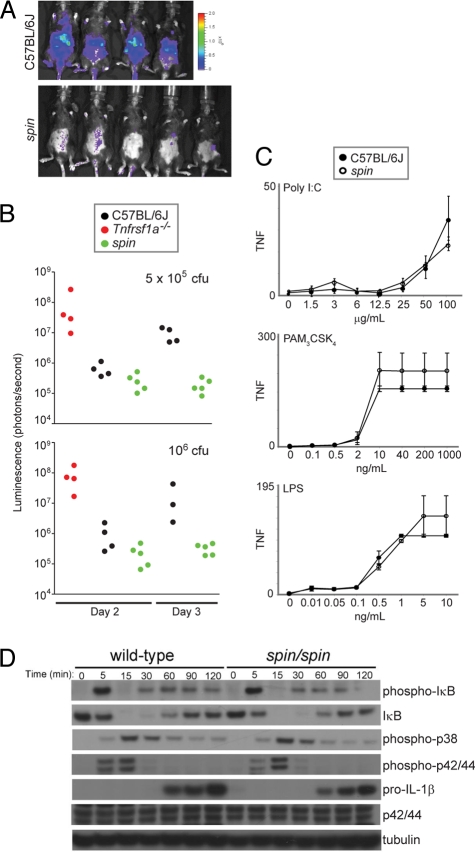
Fig. 2.
spin mice display increased resistance to L. monocytogenes. (A) Bacterial load was visualized in spin homozygotes and C57BL/6J mice 3 days after challenge with 5 × 105 cfu luminescent L. monocytogenes. Visual inspection and quantitation of luminescence demonstrated that spin homozygotes had reduced levels of bacteria compared to controls. (B) C57BL/6J, homozygous spin, or Tnfrsf1a-deficient mice were challenged with 5 × 105 or 106 cfu L. monocytogenes. The bacterial load, depicted as luminescence in photons per second, was determined 2 and 3 days after infection. Tnfrsf1a-deficient mice succumbed within 3 days of infection. One-way ANOVA and post-hoc Student-Newman-Keuls test, P < 0.05 for C57BL/6J vs. spin homozygotes on day 3 after infection with 5 × 105 cfu, and P < 0.05 for spin homozygous or C57BL/6J vs. Tnfrsf1a−/− mice on day 2 after infection with 106 cfu. (C) TNF production by peritoneal macrophages isolated from C57BL/6J or spin homozygous mice was measured in vitro in response to treatment with the indicated concentrations of TLR3, TLR2/1, or TLR4 ligands (poly I:C, Pam3CSK4, or lipopolysaccharide, respectively). TNF production was comparable between C57BL6/J and spin macrophages. The average response of cells from seven C57BL6/J and seven spin homozygous mice is plotted; error bars represent SD. (D) LPS-stimulated pro-IL-1β production, IκB degradation, and phosphorylation of MAP kinases p38, p42, and p44 in bone marrow-derived macrophages from C57BL/6J or spin homozygous mice were compared by Western blotting of lysates made at the indicated timepoints after LPS treatment in vitro. WT and spin macrophages responded similarly to LPS stimulation.