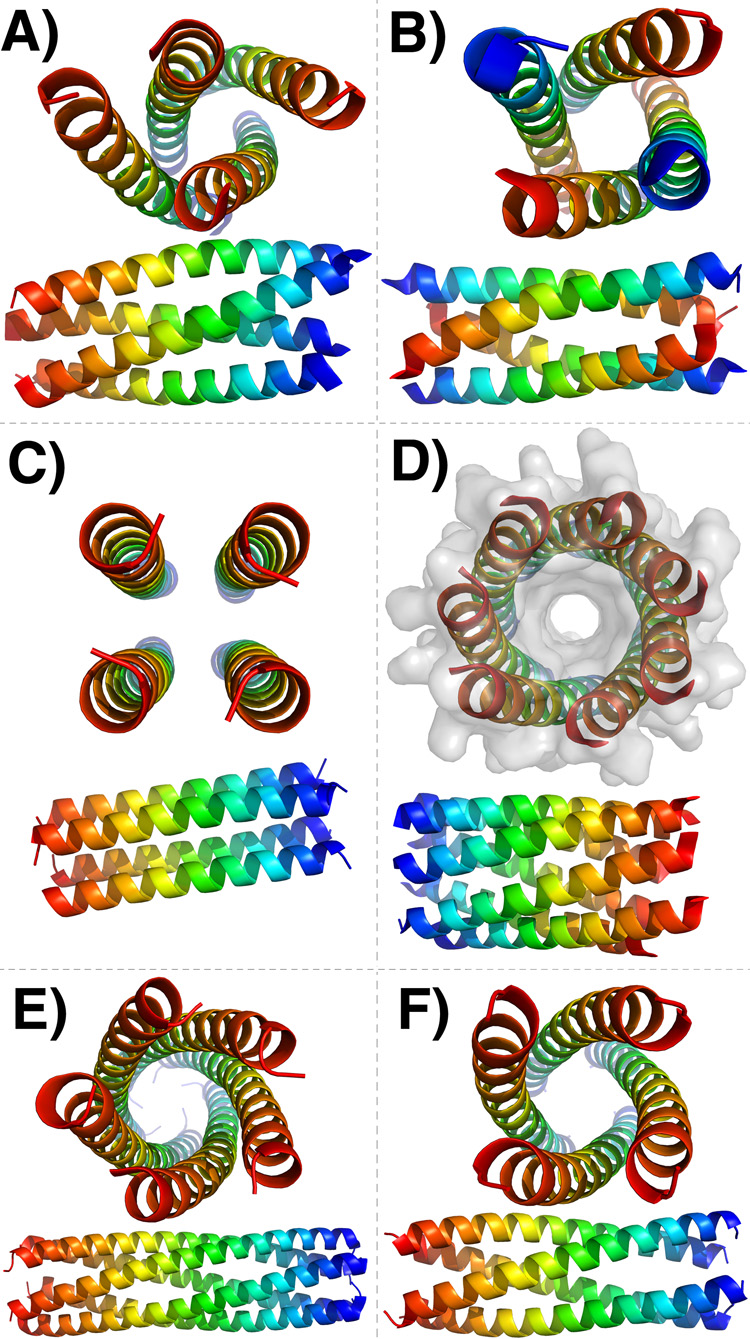
Figure 2.
New and interesting coiled-coil structures. Each coiled coil is shown axially and from the side. Color indicates helix orientation: blue – N-terminus, red – C-terminus. A, B and D Show variants of a GCN4-derived coiled coil with altered hydrophobic-polar patterning. A When all e positions are mutated to Val, departing from the canonical 3–4 repeat and creating a 3-3-1 hydrophobic repeat, the resulting sequence gives a parallel tetramer [15]. B When all g positions are substituted with either Val or Ala, also producing a 3-3-1 repeat, the result is an anti-parallel tetramer [14]. C A right-handed parallel coiled-coil tetramer [18]. D A parallel heptamer is formed when both e and g positions of GCN4 are substituted with Ala [19]. E–F A coiled coil with phenylalanine at all a and d positions folds as a parallel pentamer (E), while if just one of these phenylalanines is substituted by methionine the resulting structure is a tetramer (F) [20]