Abstract
Context: Mice deficient in prokineticin 2(PROK2) and prokineticin receptor2 (PROKR2) exhibit variable olfactory bulb dysgenesis and GnRH neuronal migration defects reminiscent of human GnRH deficiency.
Objectives: We aimed to screen a large cohort of patients with Kallmann syndrome (KS) and normosmic idiopathic hypogonadotropic hypogonadism (IHH) for mutations in PROK2/PROKR2, evaluate their prevalence, define the genotype/phenotype relationship, and assess the functionality of these mutant alleles in vitro.
Design: Sequencing of the PROK2 and PROKR2 genes was performed in 170 KS patients and 154 nIHH. Mutations were examined using early growth response 1-luciferase assays in HEK 293 cells and aequorin assays in Chinese hamster ovary cells.
Results: Four heterozygous and one homozygous PROK2 mutation (p.A24P, p.C34Y, p.I50M, p.R73C, and p.I55fsX1) were identified in five probands. Four probands had KS and one nIHH, and all had absent puberty. Each mutant peptide impaired receptor signaling in vitro except the I50M. There were 11 patients who carried a heterozygous PROKR2 mutation (p.R85C, p.Y113H, p.V115M, p.R164Q, p.L173R, p.W178S, p.S188L, p.R248Q, p.V331M, and p.R357W). Among them, six had KS, four nIHH, and one KS proband carried both a PROKR2 (p.V115M) and PROK2 (p.A24P) mutation. Reproductive phenotypes ranged from absent to partial puberty to complete reversal of GnRH deficiency after discontinuation of therapy. All mutant alleles appear to decrease intracellular calcium mobilization; seven exhibited decreased MAPK signaling, and six displayed decreased receptor expression. Nonreproductive phenotypes included fibrous dysplasia, sleep disorder, synkinesia, and epilepsy. Finally, considerable variability was evident in family members with the same mutation, including asymptomatic carriers.
Conclusion: Loss-of-function mutations in PROK2 and PROKR2 underlie both KS and nIHH.
Screening of patients with Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism (IHH) finds that loss-of-function mutations in prokineticin 2 (PROK2) and its receptor (PROKR2) underlie both disorders, with one Kallmann proband carrying both mutations. A spectrum of reproductive phenotypes was observed ranging from absent puberty to reversal of IHH in adulthood, indicating that these mutations should be considered in all GnRH-deficient subjects with or without anosmia.
Idiopathic hypogonadotropic hypogonadism (IHH) is a congenital disorder characterized by GnRH deficiency resulting in defects in sexual maturation and infertility (1). Most cases exhibit absent puberty, consistent with complete GnRH deficiency. However, some degree of pubertal development occurs in a subset of these patients (2), suggesting that underlying defects do not always prevent GnRH neuron migration and/or GnRH secretion. Furthermore, reversal of their hypogonadotropism late in adult life in 10% of cases demonstrates that maturation of the GnRH neuronal network can occur in adulthood (3). Anosmia accompanies GnRH deficiency in roughly half the patients [Kallmann syndrome (KS)] (1). Associated nonreproductive phenotypes such as cleft lip and palate, renal agenesis, and other neurological and skeletal abnormalities occur with variable frequency (4,5,6). To date, several different loci have been identified [KAL1 (GenBank accession no. M97252) (7,8), Gonadotropin Releasing Hormone Receptor (GNRHR, NM_000406) (9), Nasal Embryonic LHRH Factor (NELF, NM_015537) (10), Fibroblast Growth Factor Receptor 1 (FGFR1, BC018128) (11), G Protein Couple Receptor 54 (GPR54, AY253981) (12,13), Prokineticin 2 (PROK2, NM_021935) (14), and Prokineticin Receptor 2 (PROKR2, NM_144773) (14)], but together they only account for approximately 30% of cases, suggesting that other loci are yet to be discovered.
Prokineticin 2 (PROK2) and its receptor [prokineticin receptor 2 (PROKR2)] regulate diverse biological processes by the activation of downstream signaling pathways (15,16). PROK2 is a secreted protein of 81 amino acids, and PROKR2 is a 384 amino acid G protein-coupled receptor. The PROK2 system was initially studied for its involvement in gastrointestinal smooth muscle contraction, angiogenesis, hematopoiesis, and circadian rhythms (17,18,19,20). The knockout models for both ligand and receptor revealed a previously unappreciated role of PROK2 signaling in olfactory bulb (OB) morphogenesis and sexual maturation, suggesting PROK2 and PROKR2 as strong candidate genes for human GnRH deficiency (21,22,23). Furthermore, we have recently reported loss-of-function PROK2 mutation in an IHH pedigree with variable olfactory phenotypes (23). In addition, frame shifting and missense mutations in PROK2 and PROKR2 have been identified in a large cohort of probands with KS and variable expressivity of both olfactory and reproductive phenotypes within family members carrying the same gene defect were reported, however, no functional studies were performed on these mutations (9,14).
Therefore, we aimed to investigate further the role of PROK2/PROKR2 in the pathogenesis of KS and normosmic IHH (nIHH), study the prevalence of mutations, define the bioactivity of the mutant proteins in vitro, and characterize the genotype-phenotype relationship.
Subjects and Methods
Study population
A total of 324 IHH (170 KS, 154 nIHH), including 257 males and 67 females, was studied. GnRH deficiency was characterized by: 1) absent/incomplete puberty by age 18 yr; 2) serum testosterone (T) less than or equal to 100 ng/dl in men, or estradiol (E2) less than or equal to 20 pg/ml in women in association with low or normal levels of serum gonadotropins; 3) otherwise normal pituitary function; 4) normal serum ferritin concentrations; and 5) normal magnetic resonance imaging (MRI) of the hypothalamic-pituitary region.
The control population consisted of 173 healthy Caucasian subjects from Massachusetts General Hospital (MGH) and 34 Asian controls. The study was approved by the Human Research Committee of MGH, and all subjects provided written informed consent before participation.
Phenotyping
A detailed personal and family history of pubertal development and associated nonreproductive phenotypes was obtained. A detailed physical examination was performed. Olfactory acuity was assessed by history and when possible confirmed with quantitative smell testing. A score of less than the fifth percentile based on sex and age was deemed abnormal (24). When possible, a renal ultrasound and a cranial three-dimensional MRI to assess the olfactory system were performed as previously described (25). Patients with mutations in PROK2 or PROKR2 were invited to perform an overnight 12-h frequent blood-sampling study for LH (q10′ × 12 h) after withdrawal of hormonal therapy for a suitable washout period (typically 8 wk) at the General Clinical Research Center of MGH. The pool serum was also assayed for FSH, T, E2, and inhibin B (2).
Mutational analysis
Genomic DNA was obtained from leukocytes using a standard extraction procedure. The coding region and intron-exon boundaries of the PROK2 and PROKR2 genes were amplified using the PCR. The PROK2 gene is located on 3p13 (GenBank accession no. NM 021935) and consists of four exons. Screening was performed as previously described (21). The PROKR2 gene is located on 20p12.3 (GenBank accession no. NM 144772) and consists of two exons. The following primers were used: 5′-ACT GAC CCT GAA AGA GCA GAA GGT-3′; 5′-AGC CTG TCA GAG CCT TAA ATG AGG A-3′; 5′-TGA GGA TTC ACT GTG CCA CT-3′; 5′-TTG CGA ATC TGC TCC GTC TG-3′; 5′-TCT TTG GTG TCG AGT TCG TG-3′; and 5′-ACA CAG ATG TCA TTT CCC ATG C-3′. All amplified products were sequenced using the AmpliTaq Dye Terminator Cycle Sequencing kit and an ABI PRISM 377 DNA sequencer (Perkin-Elmer Corp., Foster City, CA). All sequence variations were found on both strands and confirmed in a separate PCR reaction. All nucleotide changes were assessed for their presence in the National Center for Biotechnology Information database of single-nucleotide polymorphisms and the expressed sequence tags database, and among control alleles. In addition, conserved across species of the mutated residue was also assessed. All genes and proteins are described using standard nomenclature (26).
Each proband carrying a mutation in PROK2/PROKR2 was also screened for other known loci involved in IHH [KAL1 (27), FGFR1 (28), GPR54 (13), NELF (10), and GnRHR (29).
In vitro studies
Generation of mutant PROK2 proteins and PROKR2 expression plasmids
Mutations in the mature form of PROK2 were introduced into the wild-type (WT) 6xHis-tagged cDNAs in a modified pGEX-3X vector (Amersham Pharmacia Biotech, Piscataway, NJ) using the Quick Change XLII Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) (15). PROK2 constructs were expressed in BL21 Escherichia coli cells, and tagged proteins were purified from cell lysates by affinity chromatography on Ni-NTA beads (QIAGEN, Inc., Valencia, CA). After digest with factor Xa to remove the tag, the proteins were renatured as previously described. Final purification was performed on reverse phase-HPLC, and the purified product was analyzed by electrospray ionization mass spectrometry using a 6.5-T HiResESI Fourier Transform mass spectrometer (IonSpec, Irvine, CA). The 27 amino acid truncated mutant (I55fsX1) was chemically synthesized as previously reported (21).
Mutations in the coding sequence of PROKR2 were introduced to a V5 C-terminal tagged WT PROKR2 cDNA (16) cloned in pCDNA3.1/V5HisD TOPO expression vector (Invitrogen Corp., Carlsbad, CA) using site-directed mutagenesis as described previously. All constructs were verified by direct nucleotide sequencing.
Ca2+ mobilization assay
The ability of PROK2 mutants to activate intracellular Ca2+ mobilization was assessed using an aequorin-based luminescent assay as previously described (16). The same protocol was used to study the PROKR2 mutants except that Chinese hamster ovary (CHO) cells were transiently transfected with WT or mutant PROKR2 plasmids using Lipofectamine (Invitrogen).
Gene transcription reporter assay
To evaluate the ability of PROKR2 mutants to activate downstream gene transcription, we developed a luciferase reporter assay using the murine early growth response (Egr)-1 gene promoter, a target of the MAPK signaling cascade (30). The Egr-1 promoter region (−1023 to +1) was amplified by PCR from mouse genomic DNA and inserted into the pGL3-basic vector (31). Using Effectene reagent (QIAGEN), subconfluent HEK293 cells in 48-well plates were transiently transfected with 5 ng WT or mutant PROKR2 plasmid, together with 5 ng Egr-1-luciferase reporter and 140 ng empty vector). Twenty-four hours after transfection, cells were stimulated with increasing doses of WT PROK2 (10−6-10−12 m range) in serum free medium containing 0.1% BSA. After 16-h incubation, the cells were lysed in Passive Lysis Buffer (Promega Corp., Madison, WI), and luciferase induction was analyzed using a Luciferase Assay System (Promega).
Data analysis
All reporter assays were performed in triplicate and repeated at least three times. The activity of the mutant at each dose was expressed as a percentage of the maximum WT response, and a four-parameter logistical dose-response curve was fitted using Prism 4 software (GraphPad Software Inc., San Diego, CA). This curve was used to determine the activity of each mutant at the EC50 dose of the wild type (range 15–50 nm), and comparisons to WT activity were made using a one-way ANOVA analysis with the Dunnett’s multiple comparison test.
Western analysis of PROKR2 mutants
For expression studies, HEK293 cells were transfected with 100 ng WT or mutant PROKR2 cDNAs as described previously. Thirty six hours after transfection, cells were lysed in Cell Lytic-M buffer (Sigma-Aldrich, St. Louis, MO) and subjected to reduced SDS-PAGE analysis using 10% Tris-HCl Ready Gel (Bio-Rad Laboratories). Resolved proteins were transferred onto polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA) in the presence of 10% methanol. After blocking in Tris-buffered saline supplemented with 5% dry milk and 0.2% Tween 20, blots were probed with anti-V5 antibodies (1:5000 dilution; Invitrogen), and immunoreactivity was visualized using Western Lighting chemiluminescence reagent (PerkinElmer, Boston, MA). To control for equal loading, the blots were then stripped using Restore Western Blot Stripping Buffer (Pierce, Rockford, IL), and reprobed with horseradish peroxidase-conjugated anti-b-actin antibody (1:10,000; Abcamat). Intensity of the immunoreactivity was quantified by densitometry, and the ratio of PROKR2 mutant to β-actin was calculated and compared with wild type. These experiments were performed at least two times.
Results
Mutations in PROK2 and PROKR2 in humans with GnRH deficiency
PROK2
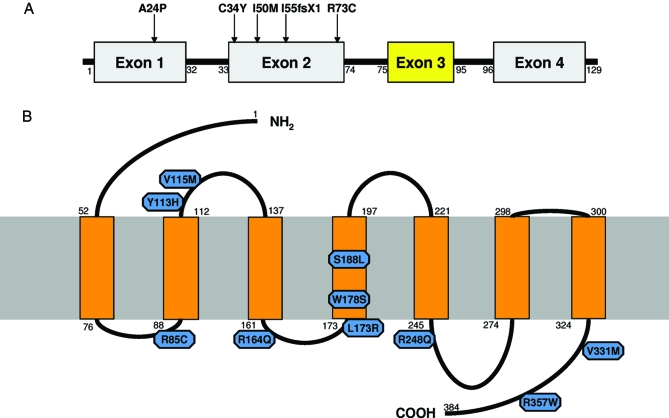
Of 170 KS and 154 nIHH, we identified five mutations in the PROK2 gene in four KS (2%) and one nIHH (<1%) probands (Tables 1 and 2 and Fig. 1). One mutation was a homozygous deletion [(p.I55fsX1+p.I55fsX1) (c.163delA +c.163delA)] previously reported (21), and the four remaining were heterozygous missense mutations: p.A24P (c.70 G>C) located in the signal peptide; p.C34Y (c.101 G>A); p.I50M (c.150 C>G); and p.R73C (c.217 C>T). The p.R73C mutation has previously been reported in a heterozygous (14) and homozygous state (32). In addition, one heterozygous synonymous change was also identified in a male proband [p.A28A (c.84 C>G)].
Table 1.
Genetic and clinical studies in IHH subjects with PROK2 and/or PROKR2 mutations
| Mutation | Dx | Sex | Puberty | Sense of smell | MRI OB | FH | Associated phenotypes | |
|---|---|---|---|---|---|---|---|---|
| Subjects with PROK2 mutations | ||||||||
| Subject no. 1 | I55fsX1a | KS | M | A | <5th percentile | A | + | Epilepsy |
| Subject no. 2 | I50M | KS | M | NA | Anosmicb | NA | NA | Synkinesia, cryptorchidism |
| Subject no. 3 | C34Y | KS | M | A | <5th percentile | NA | + | None |
| Subject no. 4 | R73C | nIHH | F | A | Normalb | NA | + | DM, osteoporosis |
| Subjects with PROKR2 mutations | ||||||||
| Subject no. 5 | Y113H | KS | F | A | Anosmicb | A | − | NA |
| Subject no. 6 | R164Q | KS | M | A | <5th percentile | NA | − | Cryptorchidism |
| Subject no. 7 | L173R | KS | M | A | <5th percentile | A | − | Pes Planus, pectus excavatum, synkinesia |
| Subject no. 8 | L173R | KS | M | A | <5th percentile | NA | − | Cryptorchidism |
| Subject no. 9 | R357W | KS | M | A | <5th percentile | A | − | None |
| Subject no. 10 | V331M | KS | M | A | <5th percentile | A | + | Pes planus, pectus excavatum, synkinesia |
| Subject no. 11 | W178S | nIHH | M | A | 73rd percentile | NA | + | Pes planus, hyperlaxity of digits, fibrous dysplasia, hearing loss |
| Subject no. 12 | S188L | nIHH | M | A | Normalb | NA | + | None |
| Subject no. 13 | R248Q | nIHH | M | P | Normalb | NL | − | Epilepsy |
| Subject no. 14 | R85C | nIHH | F | P | 23rd percentile | NL | + | None |
| Subject with combined PROK2 and PROKR2 mutations | ||||||||
| Subject no. 15 | A24P (L) | KS | F | A | <5th percentile | A | Unknown | Short 4th MC, pes planus, learning disability, strabism, hearing loss, sleep disorder |
| V115M (R) |
A, Absent; DM, diabetes; DX, diagnosis; F, female; FH, family history; L, ligand PROK2; M, male; MC, metacarpal; NA, nonassessed; NL, normal; P, partial; R, receptor PROKR2.
Homozygous mutation.
By history.
Table 2.
Genotype-phenotype relation in IHH subjects with a PROK2 and/or PROKR2 mutation
| Diagnosis | Puberty | LH secretion | Egr1-LUC assay | Calcium influx | Expression | ||
|---|---|---|---|---|---|---|---|
| PROK2 mutations | |||||||
| Subject no. 1 | I55fsX1 | KS | A | Undetectable | - | ⇊ | - |
| Subject no. 2 | I50M | KS | A | NA | - | = | - |
| Subject no. 3 | C34Y | KS | A | Undetectable | - | ⇊ | - |
| Subject no. 4 | R73C | nIHH | A | Undetectable | - | ↓ | - |
| PROKR2 mutations | |||||||
| Subject no. 5 | Y113H | KS | A | Undetectable | ⇊ | ⇊ | ↓ |
| Subject no. 6 | R164Q | KS | A | Undetectable | ⇊ | ⇊ | = |
| Subject no. 7 | L173R | KS | A | Undetectable | ⇊ | ⇊ | ↓ |
| Subject no. 8 | L173R | KS | A | Undetectable | ⇊ | ⇊ | ↓ |
| Subject no. 9 | R357W | KS | A | Undetectable | ↓ | = | |
| Subject no. 10 | V331M | KS | A | Undetectable | = | ↓ | = |
| Subject no. 11 | W178S | nIHH | A | 30 IU/liter | ⇊ | ⇊ | ↓ |
| Subject no. 12 | S188L | nIHH | A | Undetectable | ⇊ | ⇊ | ↓ |
| Subject no. 13 | R248Q | nIHH | P | Undetectable | = | ↓ | = |
| Subject no. 14 | R85C | nIHH | P | 10.9 IU/liter | ↓ | = | = |
| PROK2 and PROKR2 mutations | |||||||
| Subject no. 15 | A24P (L) | KS | A | Undetectable | - | - | - |
| V115M (R) | ⇊ | ⇊ | ↓ |
A, Absent; L, ligand PROK2; dash (-), nonassessed; NA, nonassessed; P, partial; R, receptor PROKR2.
Figure 1.
PROK2 and PROKR2 mutations. A, Schematic of the PROK2 gene. Mutations identified in the present cohort are identified with an arrow. Exons 1, 2, and 4 comprise the regular form of PROK2 (81 amino acids); the long form splice variant contains exons 1–4 (102 amino acids). B, Schematic of predicted PROKR2 protein using the SOSUI secondary structure prediction program. Mutations identified in this study are shown in blue.
PROKR2
There were 10 heterozygous missense PROKR2 mutations spanning all domains of the receptor identified in seven KS (4%) and four nIHH (3%): p.R85C (c.253 C>T), p.Y113H (c.337 T>C), p.V115M (c.343 G>A), p.R164Q (c.491 G>A), p.L173R (c.518 T>G), p.W178S (c.533 G>C), p.S188L (c.563 C>T), p.R248Q (c.743 G>A), p.V331M (c.991 G>A), and p.R357W (c.1069 C>T). The mutation L173R was seen in two unrelated probands. Subject no. 15 carries both a mutation in the ligand (p.A24P) and the receptor (p.V115M). Two heterozygous synonymous changes were also identified [p.P163P (c.489 A>G) and p.V237V (c.711 C>T)] in two unrelated KS male probands. None of these mutations was found in 346 alleles from control subjects. The p.R164Q, p.L173R, p.W178S, and p.V331M PROKR2 missense mutations have been previously reported in a cohort of KS probands (14).
No additional mutations were found in KAL1, FGFR1, GPR54, NELF, or GnRHR in probands with PROK2/PROKR2 mutations.
In vitro studies
PROK2 mutants
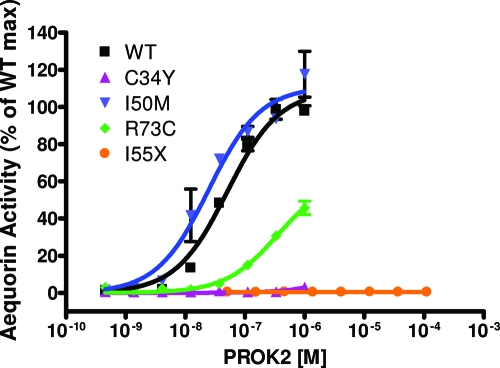
We examined the signaling properties of four PROK2 mutants (C34Y, I50M, R73C, and I55X) in an aequorin-based luminescence assay to evaluate intracellular calcium mobilization (Fig. 2). The full-length human PROK2 activated PROKR2 at a low concentration (EC50 ∼50 nm). Mutation C34Y totally abolished the calcium mobilizing activity of PROK2 (>1000-fold reduction at the Ec50 of the wild type). R73C mutant resulted in a 7-fold decreased activity at the EC50 of wild type. The truncated PROK2-27AA (p.I55fsX1) could not activate PROKR2 even at very high concentrations (>200 μm) (21). Of note, the alteration from isoleucine to methionine (I50M) (both hydrophobic amino acids) had a comparable potency as the WT PROK2. The A24P mutant was not assessed in the assay because the mutated residue is found within the signal peptide region.
Figure 2.
PROK2 mutants (C34Y, I55fsX, and R73C) are loss of function. CHO cells stably expressing WT PROKR2 and aequorin reporter were treated with increasing doses of WT or mutant PROK2, and intracellular Ca2+ influx was monitored via aequorin excitation. Data are plotted as mean ± se of three independent experiments. max, Maximum.
PROKR2 mutants
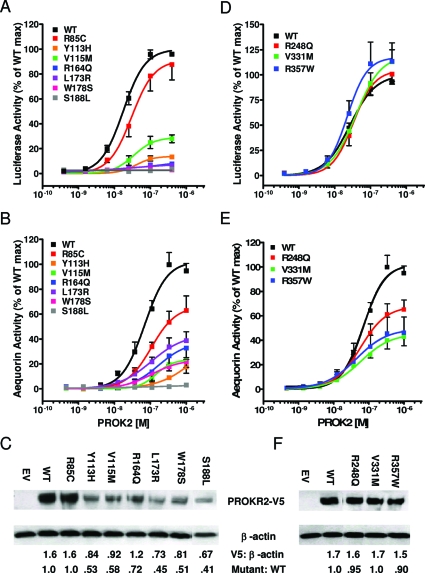
The functionality of the PROKR2 mutants was evaluated in the Egr1-LUC gene transcription assay as well as the aequorin-based Ca2+ flux assay mentioned previously. In both systems, WT receptor generated robust sigmoidal dose response curves with 10- to 15-fold and 40- to 50-fold maximum induction respectively (Fig. 3, A and B). Six PROKR2 mutants (Y113H, V115M, R164Q, L173R, W178S, and S188L) showed severely compromised activity in both assays (<40% at EC50 of wid type; P < 0.05). All six mutants demonstrated markedly reduced expression levels (40–70% of wild type; Fig. 3C), suggesting protein instability that may in part explain their poor biological function. In contrast, the R85C mutant within the first intercellular loop was moderately compromised in both assays (56 and 42% of wild type, respectively) but did not reach statistical significance in the transcription assay (P > 0.05 vs. P < 0.01 for the Ca+ assay). The expression of R85C was normal (Fig. 3C). Among the remaining mutants, R248Q located within the last intracellular loop and V331M located within the C-terminal tail had normal transcription activity but decreased activity on calcium mobilization (72 and 48% of wild type, respectively, both <0.05) (Fig. 3, D and E). Finally, the R357W exhibited higher activity compared with the wild type in the transcriptional assay (148% of wild type; P < 0.05; Fig. 3D) and reduced activity in the Ca2+ assay (60% of wild type; P > 0.05), albeit not statistically significant. The expression levels of these three mutants were comparable to the WT PROKR2 (Fig. 3F).
Figure 3.
Functional and expression analysis of PROKR2 mutants. A and D, Activity of PROKR2 mutants in transcription assay. HEK293 cells were transiently transfected with Egr1-lucifarase reporter and PROKR2 mutant constructs, and luciferase activity was monitored at increasing doses of PROK2. B and E, Activity of PROKR2 mutants in Ca2+ influx assay. CHO cells stably expressing the photoprotein aequorin were transiently transfected with WT or mutant PROKR2 plasmids, and aequorin luminescence was recorded after stimulation with PROK2. For both assays, data are presented as means ± se of three independent experiments, expressed as percentage of maximal (max) response of WT PROKR2. C and F, Western analysis of PROKR2 mutants. HEK293 cells were transiently transfected with WT or mutant PROKR2 plasmids, and equal amounts of whole cell lysate were analyzed by reduced SDS-PAGE. PROKR2 expression was visualized using anti-V5 antibodies and normalized to β-actin expression. “Mutant: WT” denotes the ratio of protein expressed.
Clinical manifestations associated with mutations in PROK2 and PROKR2
Reproductive phenotyping
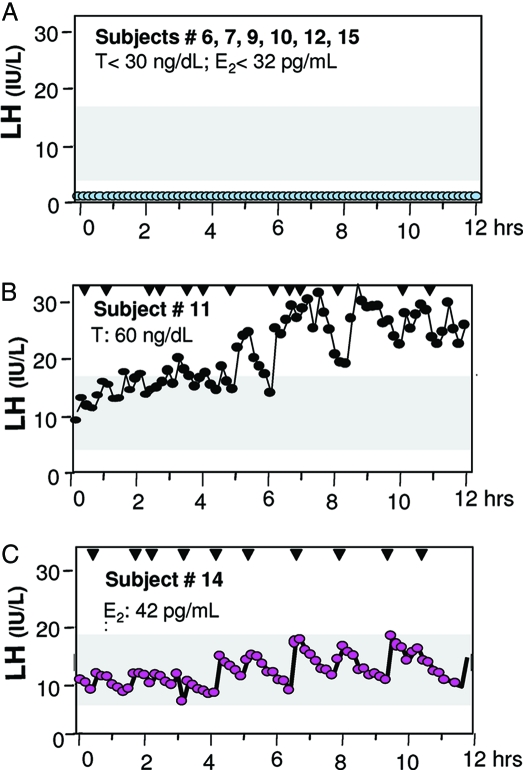
Four probands with PROK2 mutations, eight of 10 with PROKR2 mutations, and one proband carrying both a PROK2 and PROKR2 mutation, all exhibited absent puberty, and most of them had undetectable serum LH levels (Fig. 4A and Table 2). However, one proband with absent puberty (no. 11) exhibited a pulsatile pattern of LH and had elevated LH (32 IU/liter) and FSH (27 IU/liter) levels in the setting of hypogonadal T levels (Fig. 4B). This LH secretion pattern is clearly hypergonadotropic but not in the range of males with primary gonadal failure and hypogonadal serum T, suggesting an element of insufficiency of GnRH secretion as well as a primary gonadal resistance, i.e. a dual defect.
Figure 4.
Variable neuroendocrine profiles in subjects with PROK2 and PROKR2 mutations. A, Representative neuroendocrine baselines in subject nos. 6, 7, 9, 10, 12, and 15 with absent puberty. Ts for the three male subjects were all less than 30 ng/dl; E2 for subject no. 2 was less than 32 pg/ml. B, Neuroendocrine profile for subject no. 11. Baseline profiling reveals a hypergonadotropic LH secretion pattern in the setting of hypogonadal T. C, Neuroendocrine profile for female subject no. 14. Baseline profiling shows LH secretion that is elevated for her low-normal E2 level.
Two probands carrying the R248Q and R85C mutants in PROKR2 experienced partial puberty evidenced by some testicular growth in subject no. 13 (TV 6–7 ml at diagnosis) and Tanner IV breast development in subject no. 14. Interestingly, subject no. 13 experienced a reversal of his hypogonadism later in life after sex steroid therapy, as we have previously reported (3). After discontinuation of his T therapy at age 28 yr, he achieved fertility and sustained normal serum T levels thereafter. Subject no. 14 presented with primary amenorrhea, spontaneous breast development, and a normal sense of smell. She exhibited GnRH induced LH secretion (10.9 IU/liter) in the setting of a normal to hypogonadal E2 level (42 pg/ml) while still amenorrheic (Fig. 3C).
Olfactory phenotyping
Anosmia was documented by history and confirmed by formal smell testing in three of four probands with PROK2 deficiency (nos. 1–3), six of 10 probands with PROKR2 deficiency (nos. 5–10) and in the proband with both a mutation in PROK2 and PROKR2 (no. 15) (Table 1). MRI demonstrated abnormal OB and/or tract in all the KS subjects assessed (nos. 1, 5, 7, 9, 10, and 15). In contrast, a normal sense of smell by history confirmed by quantitative smell testing was documented in patient no. 4 who harbors a PROK2 mutation as well as in four IHH subjects deficient in PROKR2 (nos. 11–14) (Table 1). Two of these subjects (nos. 13 and 14) also had apparently normal olfactory structures by MRI. Thus, mutations in PROK2/PROKR2 underlie both KS and nIHH. No correlations between the olfactory phenotypes and the functionality of the mutants were apparent (Table 2).
Associated phenotypes
Several other nonreproductive phenotypes were noted in this cohort of 15 probands carrying ligand and/or receptor mutations, including epilepsy, hearing loss, synkinesia, fibrous dysplasia, sleep disorder, and other bone phenotypes (Table 1). Renal imaging performed in six probands revealed no anomalies.
Genetics in families with PROK2 and PROKR2 mutations
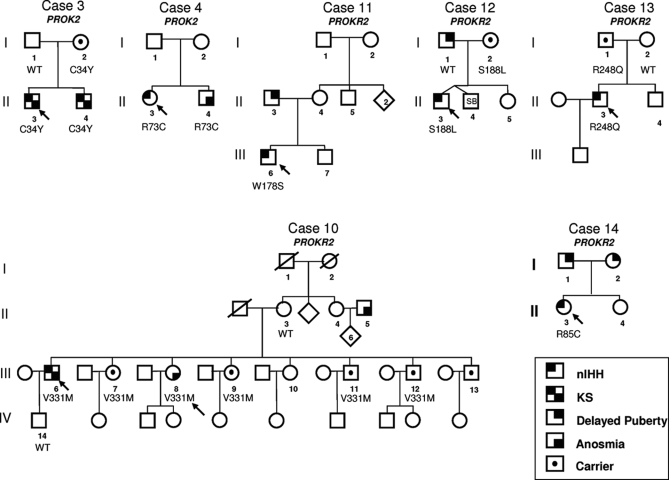
Figure 5 portrays seven familial cases with PROK2 and PROKR2 mutations. Of interest, pedigree no. 3 portrays a KS proband (case no. 3), his affected KS brother, and their asymptomatic mother, all carrying an heterozygous PROK2 mutation (p.C34Y). Pedigree no. 12 demonstrates a father with delayed puberty and an apparently asymptomatic mother who passed the PROKR2 mutation S188L on to her son (case no. 12). Pedigree no. 13 portrays a proband with nIHH harboring the PROKR2 mutation R248Q (case no. 13), which he inherited from his apparently asymptomatic father. Pedigree no. 10 demonstrates a KS proband carrying the PROKR2 mutation V331M (case no. 10) and several asymptomatic carries within the siblings (incomplete penetrance). These DNA changes in PROK2 and PROKR2 were observed in patients with GnRH deficiency and were not seen in controls, nor were they reported in the growing normative data of the database of single-nucleotide polymorphisms. The S188L mutant was clearly loss of function, whereas R248Q and V331M mutants exhibited only mild decreased calcium influx. Given the previous report of multiple gene defects synergizing to cause human GnRH deficiency (10,14), it is likely that other as-yet-to-be-described gene defects exist in these three pedigrees.
Figure 5.
Pedigree analysis reveals incomplete penetrance for PROK2 and PROKR2. Squares are males, and circles are females. The arrow denotes the proband. SB, Stillborn.
Discussion
Herein, we demonstrate that loss-of-function mutations in both PROK2 and PROKR2 contribute to human GnRH deficiency. These investigations were prompted by recent murine knockout studies of Prok2 and Prokr2 that exhibited a phenotype consistent with human KS (21,22,23). Furthermore, PROK2 and PROKR2 mutations were recently identified in a large cohort of KS patients (2 and 7%, respectively), though no functional data were provided (14). In the present study, we identified inactivating PROK2 and PROKR2 mutations not only in a large cohort of KS (frequency of 2 and 5%, respectively) but also in probands with nIHH (frequency of ∼2% for both). These data expand the phenotypical spectrum of PROK2 and PROKR2 mutations in the human, and indicate that this system plays a role in GnRH neuron development beyond OB morphogenesis.
Functional studies of mutant PROK2 demonstrate that C34Y, I55fsX, and R73C exhibit decreased intracellular calcium mobilization. The C34Y and R73C mutations alter the constitution of the conserved cysteines in the PROK2 (33) and could result in misfolding of the peptide and loss of function. The I55fsX may result in a 27AA truncated protein with no activity (21). Alternatively, this premature stop codon may result in mRNA decay and no resulting protein (34). In contrast, the mutant I50M had a similar potency as WT PROK2 for intracellular calcium mobilization, presumably because this mutation does not alter the hydrophobic property of the amino acid at this site. Nevertheless, I50M mutant may cause defects in other aspects of the PROK2 signaling pathways.
Most receptor mutants exhibit decreased expression, reduced ability to activate MAPK pathways, and reduced Ca2+ influx. Of interest, V331M, R248Q, and R357W mutants compromised Ca2+ influx despite maintaining normal or high activity in the MAPK transcription assay, suggesting that not all PROKR2 mutants activate downstream signaling pathways in the same way (Fig. 3B).
All KS probands with PROK2 or PROKR2 deficiency presented with a severe phenotype, including anosmia, absent puberty, undetectable LH levels, and a high frequency of cryptorchidism (40%), the latter indicating GnRH deficiency beginning in utero. These phenotypes are reminiscent of Prok2 and Prokr2 deficient mice, which exhibit OB dysgenesis and hypogonadotropism with immature testis and absent/severely reduced numbers of hypothalamic GnRH neurons (21,22,23). Studies of Prok2 deficient mice revealed GnRH neurons arrested between the two dysgenic OBs, a finding consistent with a migratory defect (21). Because PROKR2 and GnRH did not colocalize (21), decreased PROK2 signaling appears to impair GnRH neuron migration indirectly, likely through OB dysgenesis and/or impaired olfactory axon migration.
Here, we also demonstrate that PROK2 and PROKR2 deficiencies cause not only KS but also nIHH. In contrast to the KS probands, two of five nIHH probands exhibited partial spontaneous puberty evidenced by testicular growth (no. 13) and breast development (no. 14). Interestingly, the IHH male with partial puberty underwent spontaneous reversal of his GnRH deficiency later in life after androgen treatment. This patient with a PROKR2 mutation adds to the recent series of IHH reversal (3) and suggests that gene-environment interactions may modify a phenotype later in life. Consistently, the two corresponding mutant receptors (R248Q and R85C) displayed a mild loss of function. These human studies suggest an additional role for PROK2 signaling in GnRH neuron development/function beyond OB development. Of note, PROK2 has been a crucial “second messenger” output protein from the suprachiasmatic nucleus to various peripheral systems such as the locomotor, thermogenesis, etc. (35). Thus, it is possible that the PROK2 system contributes to the pathogenesis of nIHH in other ways such as altering GnRH pacemaking.
Our clinical investigations also suggest a role for the PROK2 system in gonadal development and function. Indeed, subject no. 11 failed to go through puberty yet exhibited a clear hypergonadotropic baseline LH secretory pattern (in the presence of hypogonadal serum T). However, these elevated gonadotropin levels are lower than expected, given the castrate T levels (T <60 ng/dl), indicating a dual hypothalamic and gonadal defect. Interestingly, both PROK2 and PROKR2 are expressed in the developing and adult testis (Genomics Institute of the Novartis Research Foundation SymAtlas; San Diego CA). The impaired GnRH secretion in this patient, albeit incomplete, is also consistent with his father’s history of delayed puberty.
Beyond their reproductive and variable olfactory phenotypes, IHH patients with deficiencies in PROK2/PROKR2 also exhibit hearing loss, synkinesia, and epilepsy with variable frequency suggesting additional developmental roles of the PROK2/PROKR2 system. Synkinesia has been reported in subjects carrying KAL1 or FGFR1 mutations (no. 1499 in Ref. 4). Generalized epilepsy in patients with PROK2/PROKR2 mutations is notable in light of decreased epileptic threshold in Prok2 −/− mice (Q.-Y.Z., unpublished data). Finally, the patient harboring both the heterozygous PROK2 and PROKR2 mutations has a history of sleep disorder, a phenotype that has been previously described in a KS patient with a heterozygous PROK2 mutation (14). Given the report of circadian anomalies in mice deficient in Prok2 (20), further studies are needed to elucidate a potential defect in the circadian rhythm in subjects harboring these mutations.
Severe GnRH deficiency in both IHH probands with heterozygous and homozygous PROK2 mutations is unusual because homozygous mutations would be expected to result in a more severe phenotype. In addition, several asymptomatic carriers and/or milder phenotypes in probands’ family members with PROK2/PROKR2 mutations were identified. Furthermore, one KS subject harbored a mutation in both PROK2 and PROKR2. These data are consistent with previous findings of an oligogenic model for IHH (10,14). Because only approximately 30% of IHH cases have a known genetic defect, we expect to document more cases of oligogenicity in IHH as novel loci are identified.
This report confirms the role of the PROK2 system in the pathogenesis of human GnRH deficiency with a wide range of both reproductive and nonreproductive phenotypes. We report mutations in both nIHH and KS indicating that the role of the prokineticin system must go well beyond that of the developing OB. Therefore, mutations in PROK2 and PROKR2 should be considered in all GnRH deficient subjects with or without anosmia. Elucidating the pathways involved in PROK2 signaling in GnRH deficient subjects will allow us to understand better its role in human reproduction.
Footnotes
This work was supported by National Institutes of Health Grants R01HD015788–21 and U54HD028138–16.
Disclosure Statement: The authors have nothing to declare.
First Published Online June 17, 2008
Abbreviations: CHO, Chinese hamster ovary; Egr, early growth response; E2, estradiol; FGFR1, Fibroblast Growth Factor Receptor 1; GNRHR, Gonadotropin Releasing Hormone Receptor; GPR54, G Protein Couple Receptor 54; IHH, idiopathic hypogonadotropic hypogonadism; KS, Kallmann syndrome; MGH, Massachusetts General Hospital; MRI, magnetic resonance imaging; NELF, Nasal Embryonic LHRH Factor; nIHH, normosmic idiopathic hypogonadotropic hypogonadism; OB, olfactory bulb; PROK2, prokineticin 2; PROKR2, prokineticin receptor 2; T, testosterone; WT, wild-type.
References
- Seminara SB, Hayes FJ, Crowley Jr WF 1998 Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocr Rev 19:521–539 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Hayes FJ, Boepple PA, DeCruz S, Seminara SB, MacLaughlin DT, Crowley Jr WF 2002 The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:152–160 [DOI] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley Jr WF, Pitteloud N 2007 Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 357:863–873 [DOI] [PubMed] [Google Scholar]
- Tsai PS, Gill JC 2006 Mechanisms of disease: insights into X-linked and autosomal-dominant Kallmann syndrome. Nat Clin Pract Endocrinol Metab 2:160–171 [DOI] [PubMed] [Google Scholar]
- Quinton R, Duke VM, Robertson A, Kirk JM, Matfin G, de Zoysa PA, Azcona C, MacColl GS, Jacobs HS, Conway GS, Besser M, Stanhope RG, Bouloux PM 2001 Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin Endocrinol (Oxf) 55:163–174 [DOI] [PubMed] [Google Scholar]
- Hardelin JP, Levilliers J, Blanchard S, Carel JC, Leutenegger M, Pinard-Bertelletto J-P, Bouloux P, Petit C 1993 Heterogeneity in the mutations responsible for X chromosome-linked Kallmann syndrome. Hum Mol Genet 2:373–377 [DOI] [PubMed] [Google Scholar]
- Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, Brown CJ, Willard HF, Lawrence C, Persico MG, Camerino G, Ballabio A 1991 A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature 353:529–536 [DOI] [PubMed] [Google Scholar]
- Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D, Bougueleret L, Delemarre-van de Waal HA, Lutfalla G, Weissenbach J, Petit C 1991 The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell 67:423–435 [DOI] [PubMed] [Google Scholar]
- de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E 1997 A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med 337:1597–1602 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W 2007 Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, Pecheux C, Le Tessier D, Cruaud C, Delpech M, Speleman F, Vermeulen S, Amalfitano A, Bachelot Y, Bouchard P, Cabrol S, Carel JC, Delemarre-van de Waal H, Goulet-Salmon B, Kottler ML, Richard O, Sanchez-Franco F, Saura R, Young J, Petit C, Hardelin JP 2003 Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet 33:463–465 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP 2006 Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet 2:e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DC, Bullock CM, Ehlert FJ, Chen JL, Tian H, Zhou QY 2002 Identification and molecular characterization of two closely related G protein-coupled receptors activated by prokineticins/endocrine gland vascular endothelial growth factor. J Biol Chem 277:19276–19280 [DOI] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY 2001 Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol 59:692–698 [DOI] [PubMed] [Google Scholar]
- Schweitz H, Pacaud P, Diochot S, Moinier D, Lazdunski M 1999 MIT(1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett 461:183–188 [DOI] [PubMed] [Google Scholar]
- Lecouter J, Lin R, Ferrara N 2004 EG-VEGF: a novel mediator of endocrine-specific angiogenesis, endothelial phenotype, and function. Ann NY Acad Sci 1014:50–57 [DOI] [PubMed] [Google Scholar]
- LeCouter J, Lin R, Ferrara N 2002 Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nat Med 8:913–917 [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY 2002 Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley Jr WF 2007 Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY 2005 Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 308:1923–1927 [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y 2006 Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA 103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Applebaum S, Zusho H, Settle RG 1985 Sex differences in odor identification ability: a cross-cultural analysis. Neuropsychologia 23:667–672 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Acierno Jr JS, Meysing A, Eliseenkova AV, Ma J, Ibrahimi OA, Metzger DL, Hayes FJ, Dwyer AA, Hughes VA, Yialamas M, Hall JE, Grant E, Mohammadi M, Crowley Jr WF 2006 Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 103:6281–6286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis SE 1998 Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum Mutat 11:1–3 [DOI] [PubMed] [Google Scholar]
- Oliveira LM, Seminara SB, Beranova M, Hayes FJ, Valkenburgh SB, Schipani E, Costa EM, Latronico AC, Crowley Jr WF, Vallejo M 2001 The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab 86:1532–1538 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Acierno Jr JS, Meysing AU, Dwyer AA, Hayes FJ, Crowley Jr WF 2005 Reversible Kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab 90:1317–1322 [DOI] [PubMed] [Google Scholar]
- Beranova M, Oliveira LM, Bedecarrats GY, Schipani E, Vallejo M, Ammini AC, Quintos JB, Hall JE, Martin KA, Hayes FJ, Pitteloud N, Kaiser UB, Crowley Jr WF, Seminara SB 2001 Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 86:1580–1588 [DOI] [PubMed] [Google Scholar]
- Schwachtgen JL, Houston P, Campbell C, Sukhatme V, Braddock M 1998 Fluid shear stress activation of egr-1 transcription in cultured human endothelial and epithelial cells is mediated via the extracellular signal-related kinase 1/2 mitogen-activated protein kinase pathway. J Clin Invest 101:2540–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy BA, Lau LF, Nathans D 1988 A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with “zinc finger” sequences. Proc Natl Acad Sci USA 85:7857–7861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Fouveaut C, Leclercq S, Jacquemont S, Boullay HD, Lespinasse J, Delpech M, Dupont JM, Hardelin JP, Dode C 2008 February 20 Biallelic mutations in the prokineticin-2 gene in two sporadic cases of Kallmann syndrome. Eur J Hum Genet [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kaser A, Winklmayr M, Lepperdinger G, Kreil G 2003 The AVIT protein family. Secreted cysteine-rich vertebrate proteins with diverse functions. EMBO Rep 4:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat LE, Carmichael GG 2001 Quality control of mRNA function. Cell 104:173–176 [DOI] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY 2006 Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci 26:11615–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]