FIGURE 4.
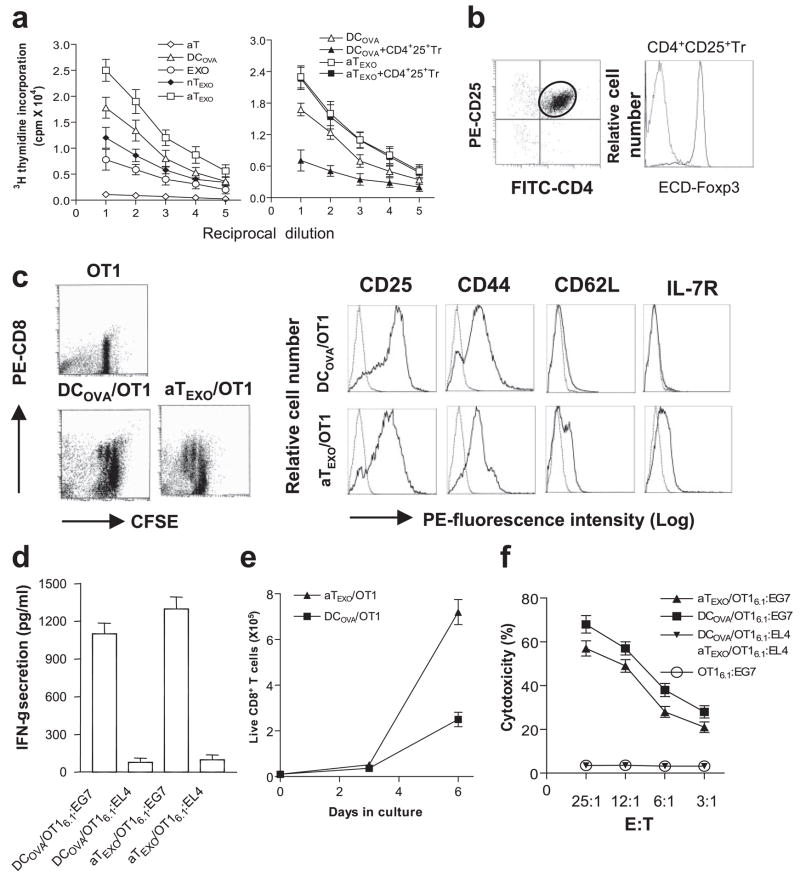
Stimulation of CD8+ memory T cell responses in vitro. a, In vitro CD8+ cell proliferation assay. EXOOVA (10 μg/ml), DCOVA, nTEXO, aTEXO, and Con A-activated OTII T (aT) cells and their 2-fold dilutions were cocultured with a constant number of OT I CD8+T cells in presence or absence of CD4+25+ Tr cells. After 3 days, the proliferation response of CD8+ T cells was determined by [3H]thymidine uptake assay. b, Phenotypic analysis of CD4+25+ Tr cells. The CD4+25+ Tr cells were purified from C57BL/6 mouse splenocytes using CD25 microbeads, stained with PE-CD25, FITC-CD4, and ECD-Foxp3 Abs, and then analyzed by flow cytometry. The FITC-CD4- and PE-CD25-positive T cells were grouped for analysis of ECD-Foxp3 expression (solid line). Irrelevant isotype-matched Ab was used as a control (dotted line). c, Phenotypic analysis of in vitro aTEXO-primed CD8+ T cells. CFSE-labeled naive T cells derived from OT I mice were primed with irradiated DCOVA and aTEXO for 2 days in vitro and stained for CD8, CD25, CD44, CD62L, and IL-7R, respectively. Dot plots of CFSE-positive CD8+ T cells stained with PE-anti-CD8 Ab are shown, indicating that the CFSE-labeled CD8+ T cells underwent some cycles of cell division, and were sorted by flow cytometry for assessment of CD25, CD44, CD62L, and IL-7R expression using PE-labeled Abs (solid lines) or PE-isotype-matched irrelevant Abs (dotted lines) by flow cytometry. d, The in vitro DCOVA- and aTEXO-activated OT I CD8+CD45.1+ T cells were purified using biotin anti-CD45.1 Ab and anti-biotin microbeads (Miltenyi Biotec) and are referred to as DCOVA/OT I6.1 and aTEXO/OT I6.1, respectively. They were then incubated with irradiated (4,000 rad) EG7 and EL4 for 24 h. The supernatants in wells containing DCOVA/OT I6.1 plus EG7 or EL4 cells (DCOVA/OT I6.1:EG7 or DCOVA/OT I6.1:EL4) and aTEXO/OT I6.1 plus EG7 or EL4 cells (aTEXO/OT I6.1:EG7 or aTEXO/OT I6.1:EL4) were examined for IFN-γ expression by ELISA. e, T cell proliferation assay. In vitro DCOVA- and aTEXO-activated CD8+CD45.1+ T cells (0.4 × 105 cells/well) derived from OT I/B6.1 mouse OTI CD8+ T cells, primed on day 0 with irradiated DCOVA (■) or aTEXO (▴), were maintained in cultures for 1 wk with the indicated cytokines (IL-2 (50 U/ml) and IL-7 (10 ng/ml)) added on days 3 and 5. Live CD8+ T cells with trypan blue exclusion for each culture done in triplicate were counted at the indicated time points. f, In vitro cytotoxicity assay. The above DCOVA/OT I6.1 (■) and aTEXO/OT I6.1 (▴) cells were used as effector cells, whereas 51Cr-labeled EG7 or EL4 cells were used as target cells in a chromium release assay. One representative experiment of three is displayed.