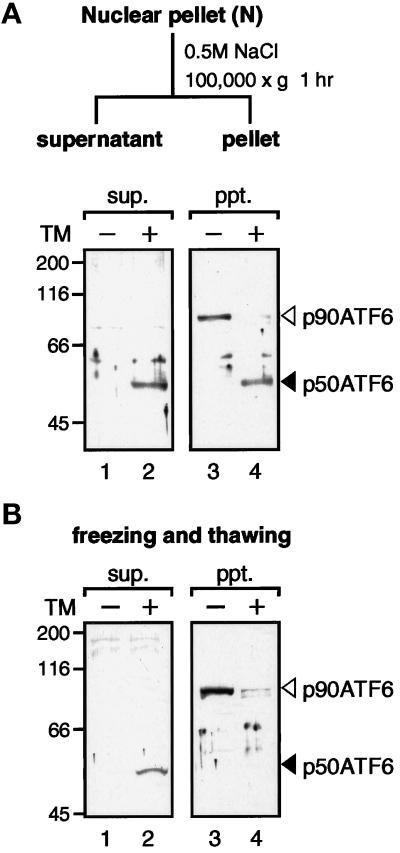
Figure 7.
Solubility of p90ATF6 and p50ATF6. (A) Fractionation of nuclear pellet. The nuclear pellet fraction prepared as described in the legend to Figure 5 was washed with PBS three times and resuspended in nuclear extraction buffer (20 mM HEPES-KOH, pH 7.6, 25% glycerol, 0.5 M NaCl, 1.5 mM MgCl2, 1 mM EDTA, 5 μg/ml pepstatin A, 5 μg/ml leupeptin, and 2 μg/ml aprotinin). After rotating for 1 h at 4°C, the samples were centrifuged at 100,000 × g for 1 h to separate the supernatant (sup.) from the pellet (ppt.). Aliquots of the indicated fractions were subjected to SDS-PAGE (10% gel) and analyzed by immunoblotting with anti-ATF6 antibody. The positions of p90ATF6 and p50ATF6 are marked as in Figure 1. (B) Effect of freezing and thawing. HeLa cells cultured in 60-mm dishes until 80% confluency were incubated in the absence (−) or presence (+) of 2 μg/ml tunicamycin (TM) for 4 h. Cells were washed with PBS, scraped with a rubber policeman, and centrifuged at 1000 × g for 5 min. After three cycles of freezing and thawing of cell pellets suspended in 50 μl of PBS, samples were centrifuged at 15,000 × g for 10 min to separate the supernatant (sup.) from the pellet (ppt.), which was then resuspended in 50 μl of PBS. Aliquots of each supernatant and pellet corresponding to 1 × 105 cells were subjected to SDS-PAGE (10% gel) and analyzed by immunoblotting with anti-ATF6 antibody.