Figure 3.
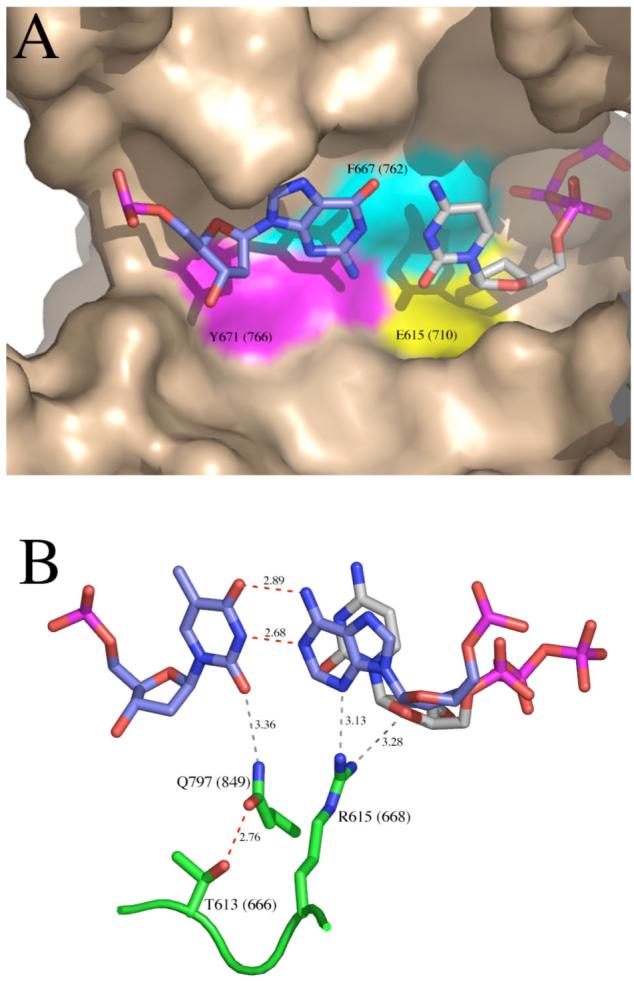
Details of the polymerase active site from ternary complex crystal structures of A-family polymerases, homologous to Klenow fragment. A. Binding pocket for the nascent base pair, illustrated using the ternary complex of Klentaq, PDB file 3KTQ (3). The templating base is colored predominantly in blue, and the incoming dNTP predominantly in gray. On the surface representation of the protein, the colored areas show the side chains homologous to E710 (yellow), F762 (cyan) and Y766 (magenta) of Klenow fragment. The remaining wall of the binding pocket is provided by the terminal base pair (not shown) which would cover the nascent base pair in the view shown here. B. Contacts around the minor groove face of the primer-terminal base pair, illustrated using the ternary complex of Bst DNA polymerase, PDB file 1LV5 (4). The terminal base pair is colored predominantly in blue, and the incoming dNTP predominantly in gray. Protein side chains discussed in the text are shown in green; T613, R615 and Q797 of Bst DNA polymerase are homologous to T666, R668 and Q849 of Klenow fragment. Hydrogen bonds shown in red are predicted to be strong and those in gray weaker. These figures were constructed using PyMOL (DeLano Scientific).
