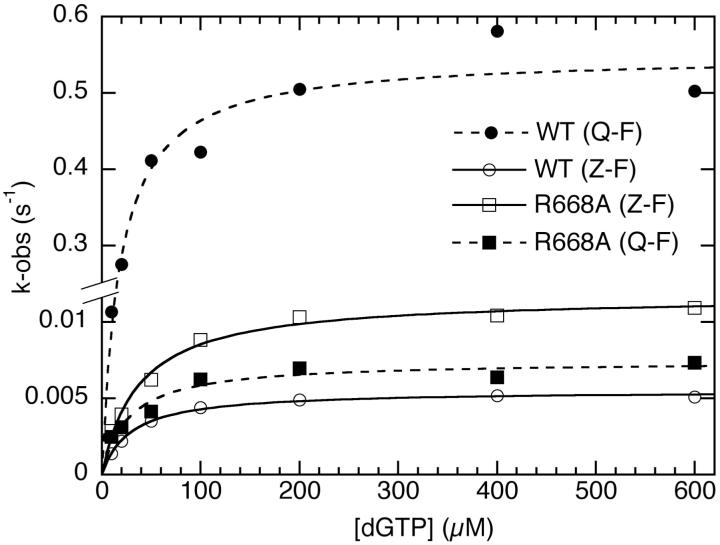
Figure 4.
Kinetic demonstration of the requirement for a hydrogen bond from R668 to the minor groove of the primer terminus. Q at the primer terminus, which is capable of hydrogen bonding, is extended much more readily by wild-type Klenow fragment than Z, which has no hydrogen-bonding groups. (Note the change in scale on the vertical axis.) The R668A mutation removes the proposed hydrogen bond donor on the protein and essentially abolishes the distinction between primer-terminal Q and Z.