FIG. 4.
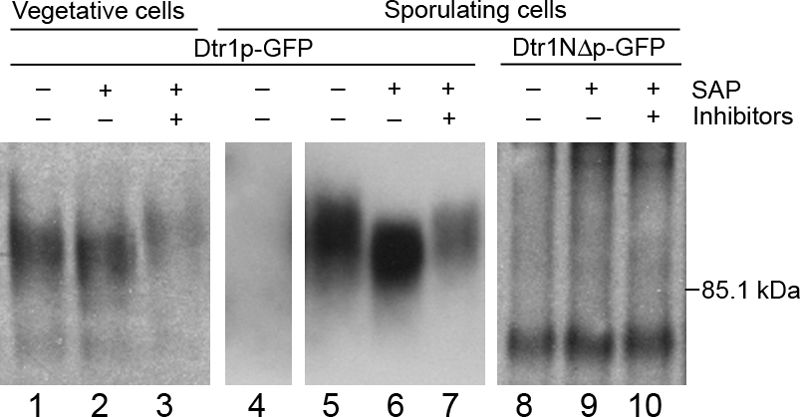
Dtr1p-GFP is a phosphoprotein. CLB2-driven Dtr1p-GFP extracted from vegetative cells (Y7479) (lanes 1 to 3), untagged Dtr1p extracted from sporulating cells (Y6582) (lane 4), and Dtr1p-GFP extracted from sporulating cells (Y6583) (lanes 5 to 7) were treated with (+) or without (−) SAP in the presence (+) or absence (−) of phosphatase inhibitors and subjected to SDS-PAGE followed by immunoblot analysis. Dtr1p-GFP showed a change in mobility following treatment with SAP that was blocked in the presence of inhibitors, indicating that Dtr1p is a phosphoprotein. Analysis of Dtr1NΔp-GFP (Y7419) (lanes 8 to 10) suggests that the change in mobility observed on SDS-PAGE is due to phosphorylation of the N-terminal cytoplasmic domain.
