FIG. 7.
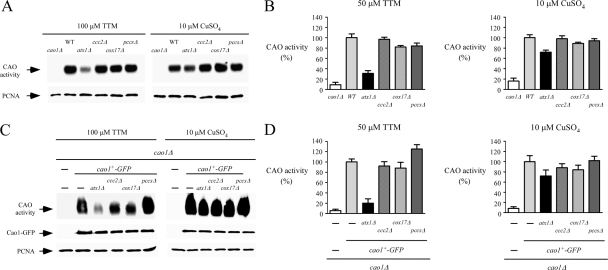
Production of fully active Cao1 requires functional Atx1. (A) Logarithmic-phase cultures of isogenic FY435 (WT), cao1Δ, atx1Δ, ccc2Δ, cox17Δ, and pccsΔ strains were treated in the presence of TTM (100 μM) or CuSO4 (10 μM) at 30°C. After 8 h of treatment, cell lysates were prepared from each culture and analyzed using an in-gel peroxidase-catalyzed chemiluminescence assay for CAO activity (top). As an internal control, aliquots of cell lysates were probed by immunoblotting using anti-PCNA antibody (bottom). (B) CAO activity was quantitated from the cell lysates described for panel A by use of a spectrophotometric method with 4-aminoantipyrine and vanillic acid. Error bars indicate the standard deviation of activities from samples analyzed in triplicate. (C) Isogenic S. pombe strains bearing a single deletion (cao1Δ) or a double deletion (atx1Δ cao1Δ, ccc2Δ cao1Δ, cox17Δ cao1Δ, or pccsΔ cao1Δ) were transformed with an integrative plasmid expressing a functional Cao1-GFP fusion protein. Whole-cell extracts from each transformant grown in the presence of TTM (100 μM) or CuSO4 (10 μM) were prepared and analyzed for CAO activity using an in-gel assay (top). Aliquots of total-extract preparations were examined by immunoblotting using either anti-GFP (middle) or anti-PCNA (bottom) antibody. As a control, an S. pombe strain bearing a cao1 deletion was transformed with an empty integrative vector (−). (D) Total-extract preparations from strains described for panel C were analyzed by a spectrophotometric method using 4-aminoantipyrine/vanillic acid. The values of CAO activities are the means from three replicates ± standard deviations.