Figure 3.
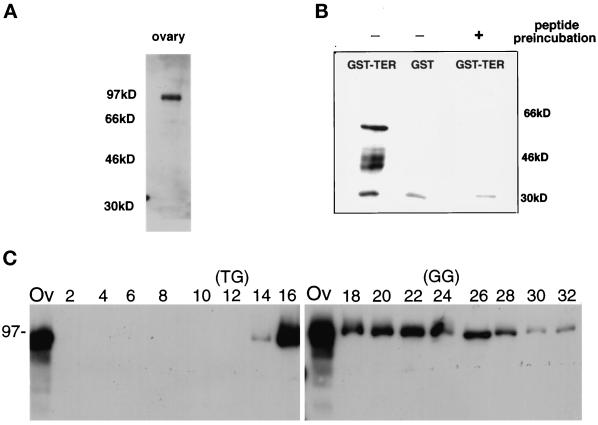
Immunological evaluation of TER94. (A) Western blot analysis of wild-type ovarian extract using antibodies raised against a TER94 peptide (MATERIALS AND METHODS) revealed a 94-kDa protein. The positions of molecular weight standards are shown on the left. (B) Immunoblot containing protein isolated from E. coli expressing either GST–TER94 ORF (lane 1; predicted molecular mass of 58 kDa) or GST alone (lane 2) was incubated with TER94 peptide antibodies (lanes 1 and 2). The multiple bands that appear near 46 kDa in lane 1 are probably breakdown products of the GST–TER94 recombinant protein. Lane 3 shows the results obtained when TER94 peptide antiserum was incubated with the antigenic peptide before incubating with a membrane carrying protein from E. coli expressing GST–TER94 ORF. The band at 30 kDa is a nonspecific reactivity because it is common in all samples and is not blocked by antigenic peptide incubation. (C) Protein was isolated from even-numbered fractions of a sucrose gradient and separated on two 10% SDS-PAGE gels. A sample of the input total ovarian protein (lane Ov) was included on each gel. The fraction in lane 2 was collected from the bottom, whereas lane 32 is from the top of the gradient. The corresponding immunoblot was incubated with TER94 antibodies to determine the fractions that contained TER94. A parallel sucrose gradient was run containing thyroglobulin (TG, Mr 670,000) and bovine gamma globulin (GG, Mr 158,000) to establish the position of known molecular weight standards.