Abstract
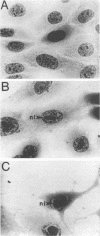
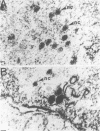
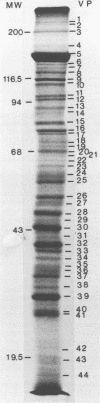
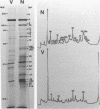
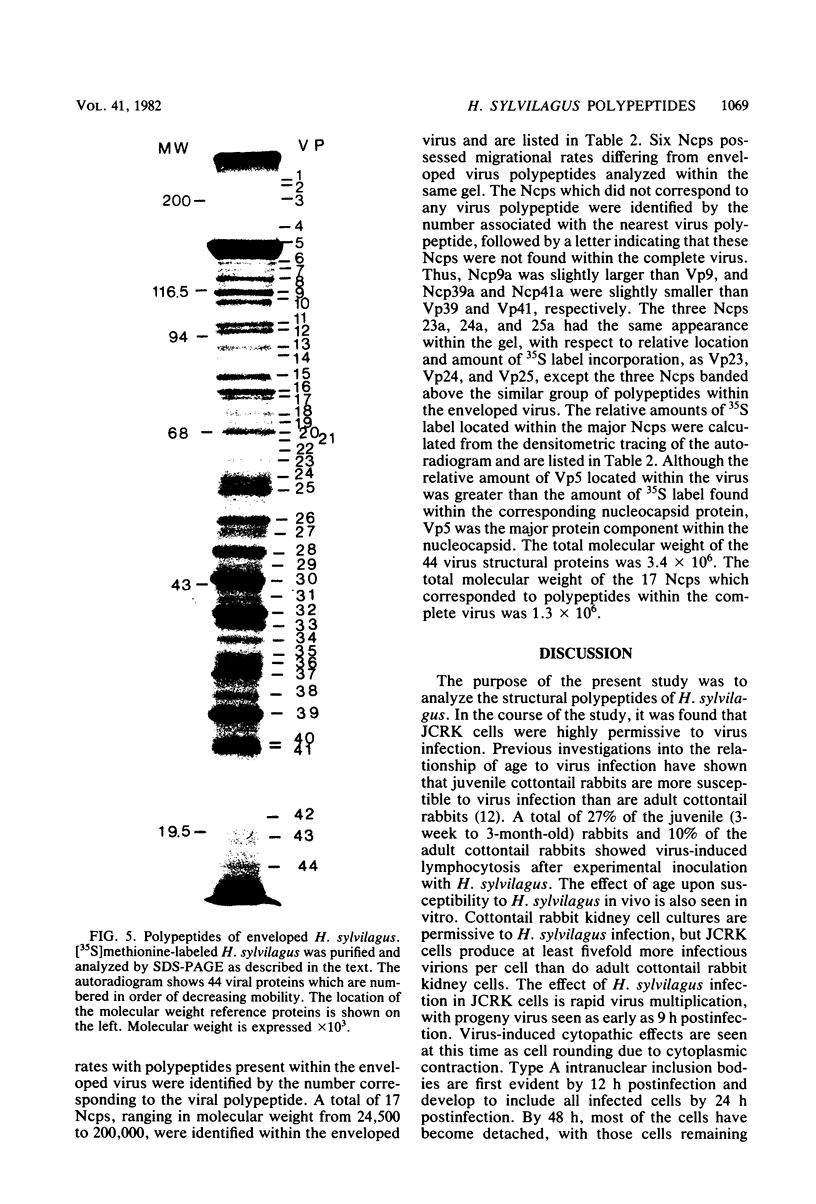
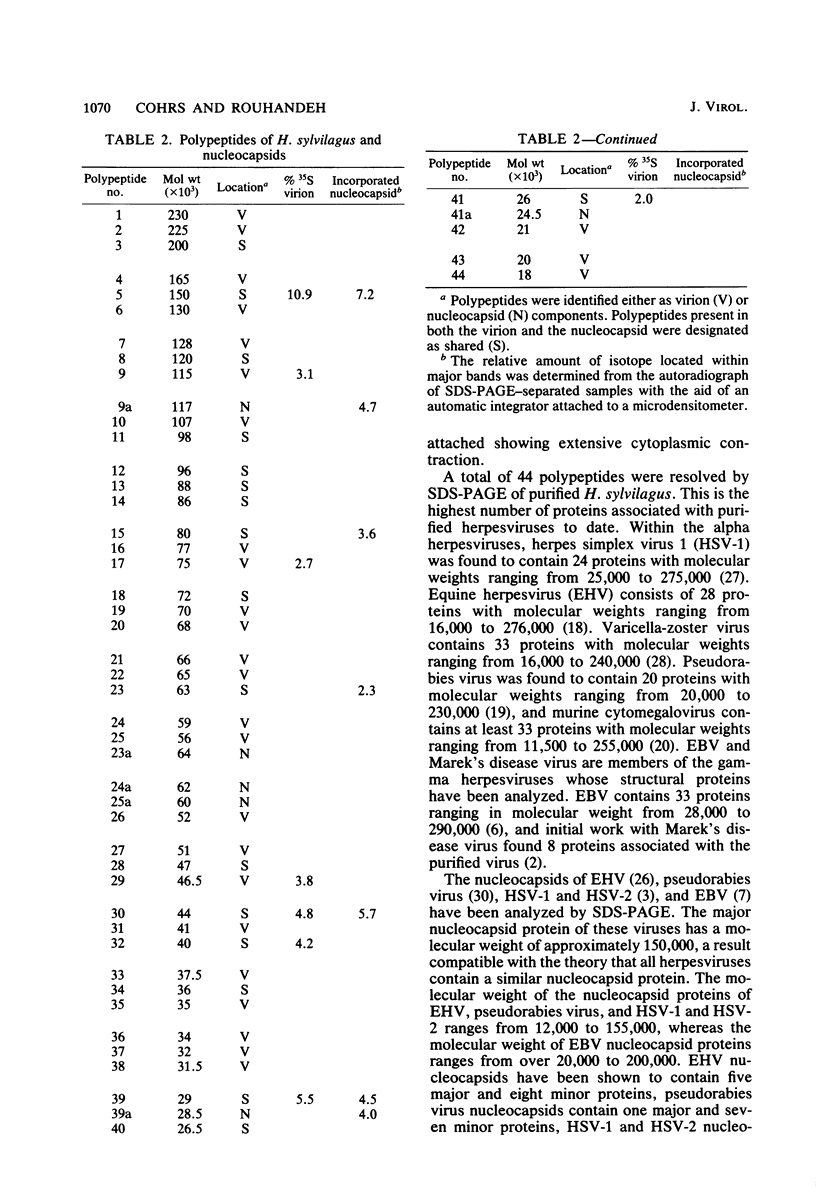
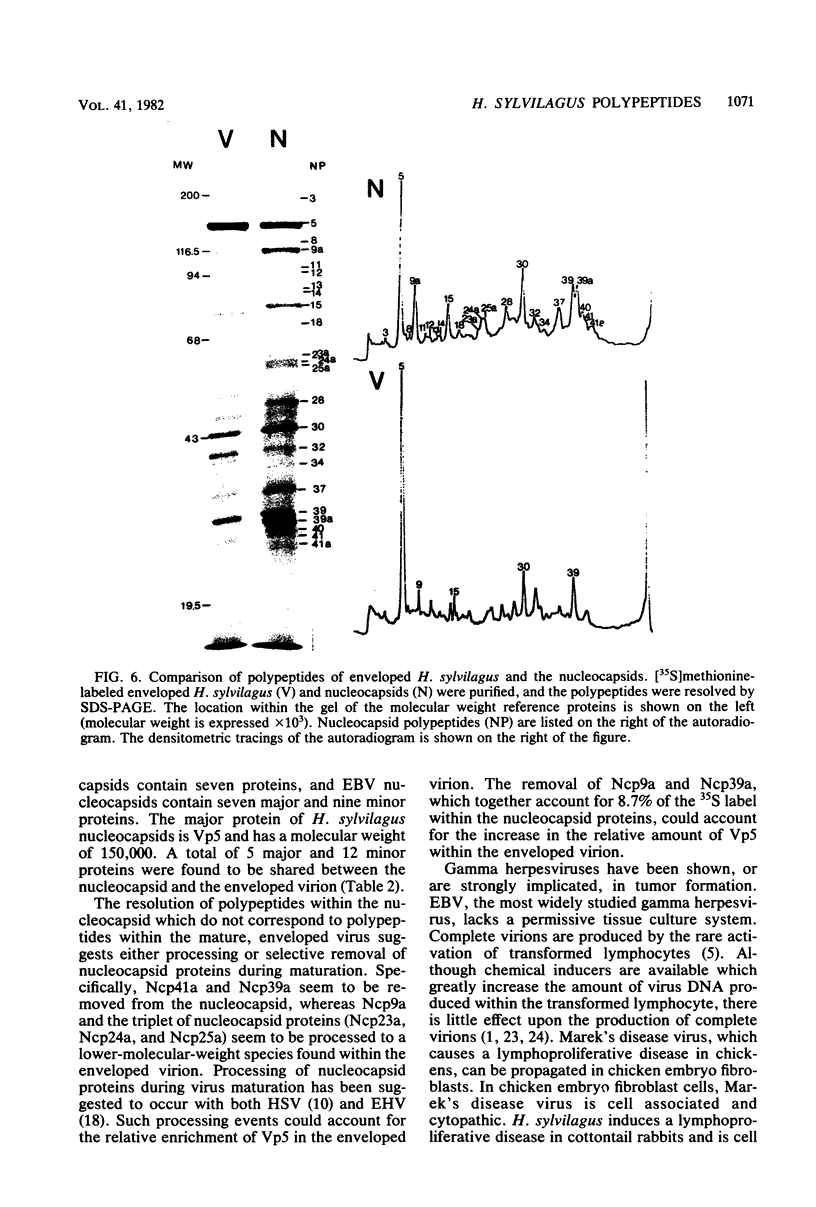
Herpesvirus sylvilagus was propagated in juvenile cotton tail rabbit kidney cells and purified from the cytoplasmic fraction of the infected cells. The purification procedure included zonal centrifugation through a 5 to 30% dextran t-10 gradient, followed by equilibrium centrifugation in a 5 to 50% potassium tartrate gradient. H. sylvilagus formed one band after centrifugation through the tartrate gradient at a density of 1.22 g/cm3. Contamination of the purified virus preparation by cellular proteins was less than 0.2% as determined by the removal of radioactivity from an artificially mixed sample containing [35S]methionine-labeled control cells and nonlabeled infected cells. H. sylvilagus nucleocapsids were isolated from infected cell nuclei and purified by sedimentation through a 36% sucrose cushion, followed by equilibrium centrifugation in 5 to 50% tartrate gradient. Forty-four polypeptides ranging in molecular weight from 18,000 to 230,00 were resolved when [35S]methionine-labeled enveloped H. sylvilagus was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Seventeen polypeptides found within the enveloped virus were also identified with the nucleocapsid. Six additional nucleocapsid polypeptides han no counterparts within the enveloped virus. The major polypeptide within both the virus and the nucleocapsid had a molecular weight of 150,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Lee L. F., Nazerian K., Burmester B. R. Structural proteins of Marek's disease virus. Virology. 1972 Feb;47(2):434–443. doi: 10.1016/0042-6822(72)90279-6. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Ponce de Leon M., Diggelmann H., Lawrence W. C., Vernon S. K., Eisenberg R. J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980 May;34(2):521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Wolff E., Kieff E. Proteins of Epstein-Barr Virus. II. Electrophoretic analysis of the polypeptides of the nucleocapsid and the glucosamine- and polysaccharide-containing components of enveloped virus. J Virol. 1976 Apr;18(1):289–297. doi: 10.1128/jvi.18.1.289-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. 8. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972 Nov;10(5):1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Hinze H. C. Morphological studies on Herpesvirus sylvilagus in rabbit kidney cell cultures. Cancer Res. 1972 Jun;32(6):1340–1350. [PubMed] [Google Scholar]
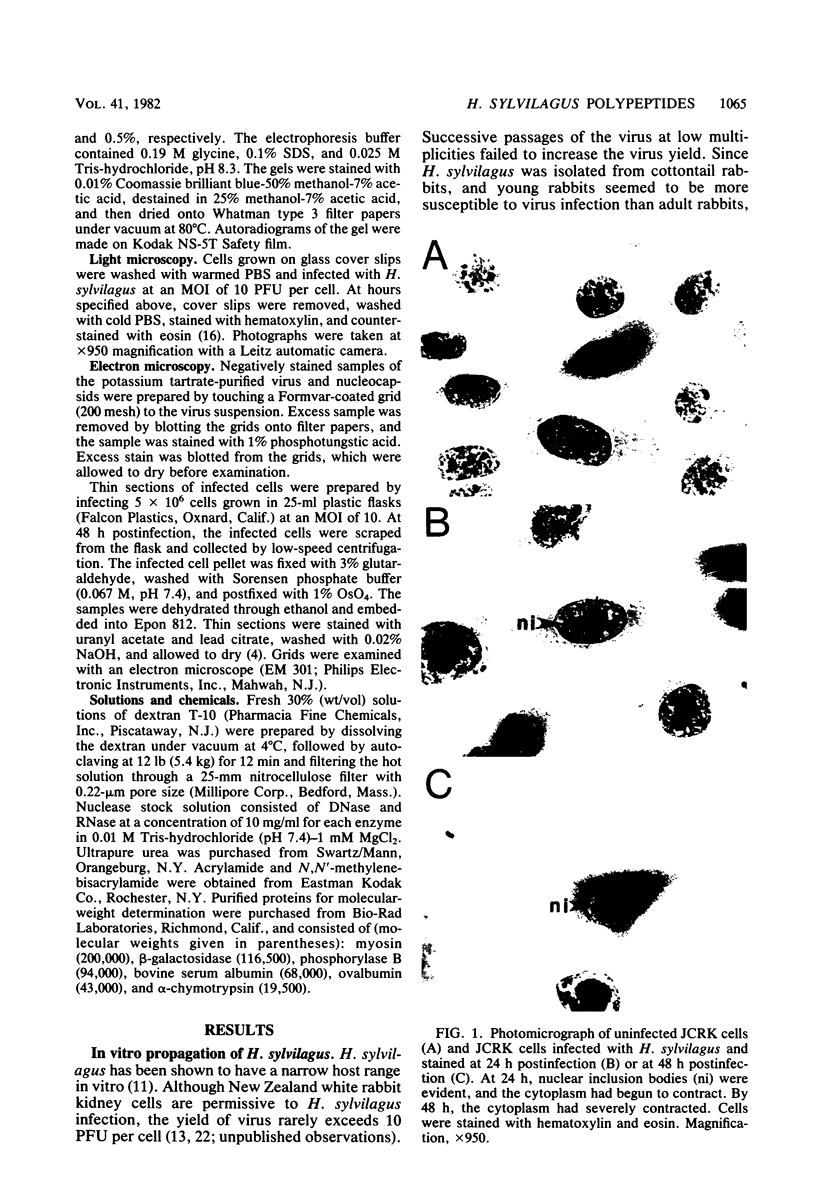
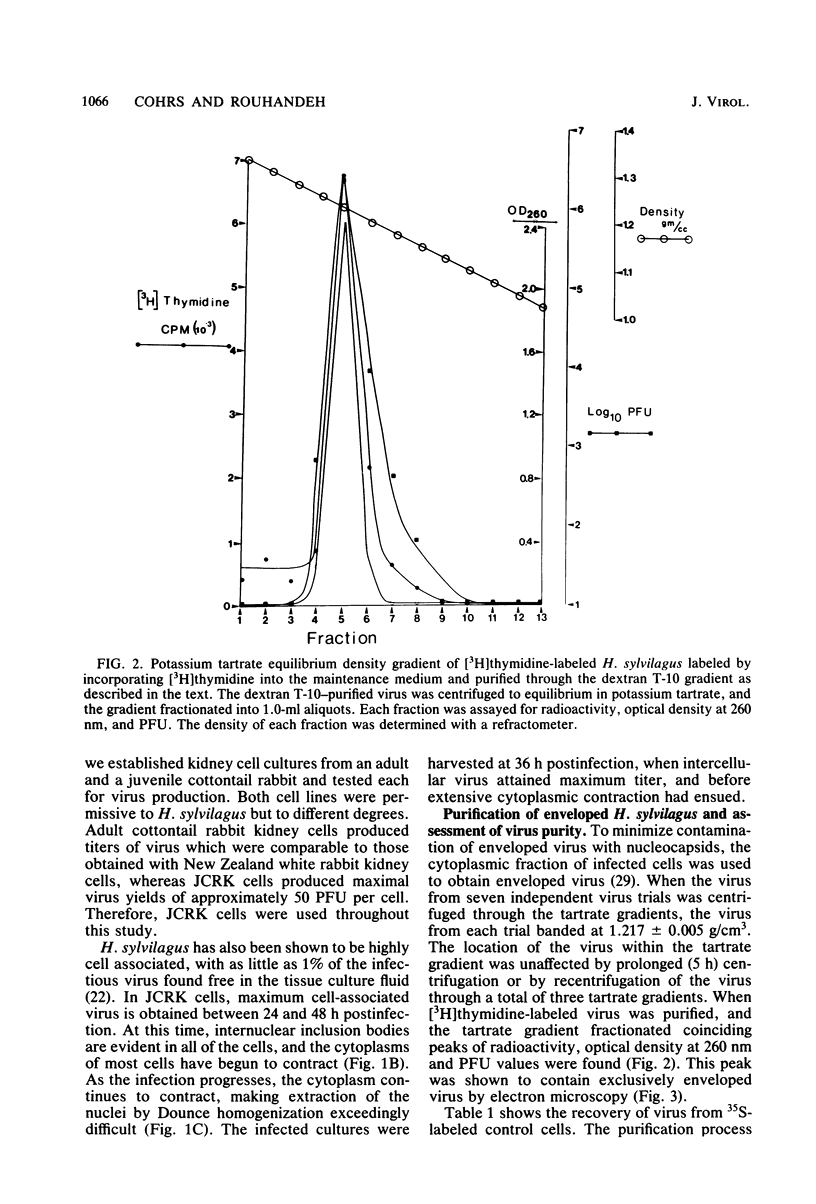
- Hinze H. C., Chipman P. J. Role of herpesviruses in malignant lymphoma in rabbits. Fed Proc. 1972 Nov-Dec;31(6):1639–1642. [PubMed] [Google Scholar]
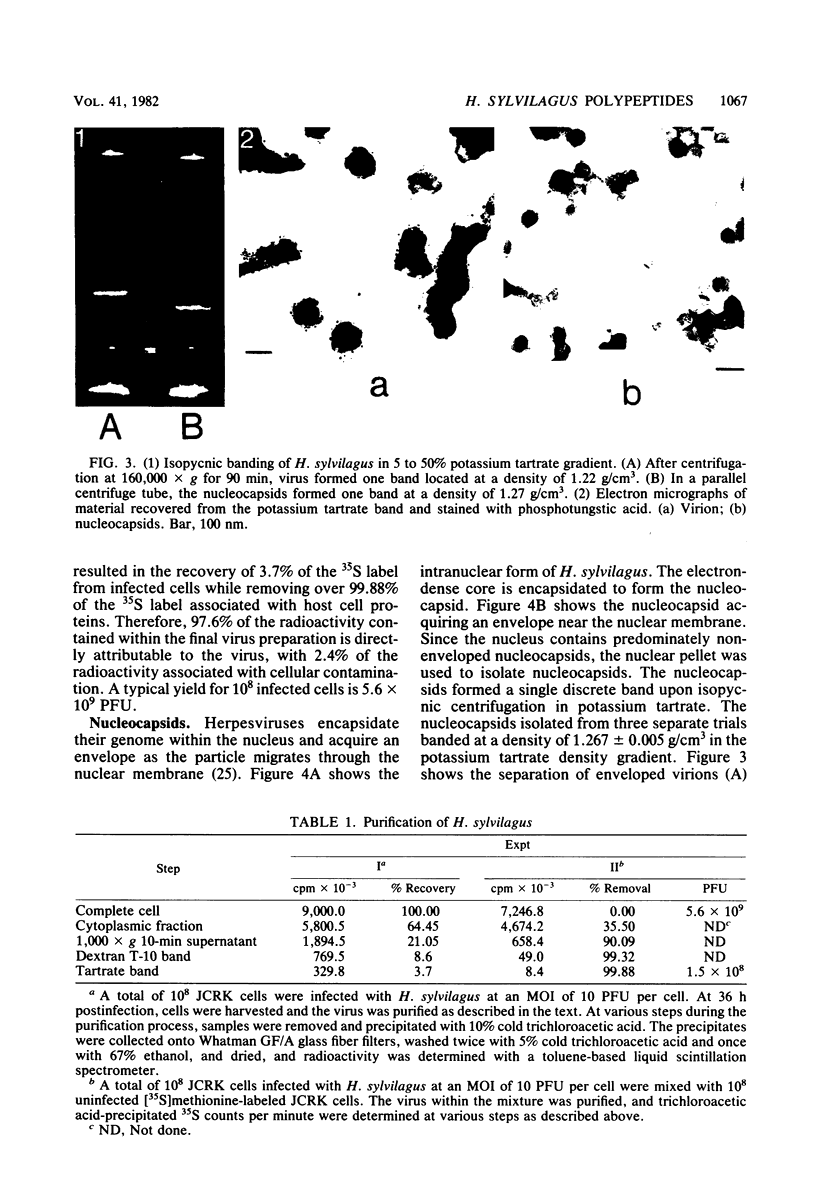
- Hinze H. C. Induction of lymphoid hyperplasia and lymphoma-like disease in rabbits by Herpesvirus sylvilagus. Int J Cancer. 1971 Nov 15;8(3):514–522. doi: 10.1002/ijc.2910080320. [DOI] [PubMed] [Google Scholar]
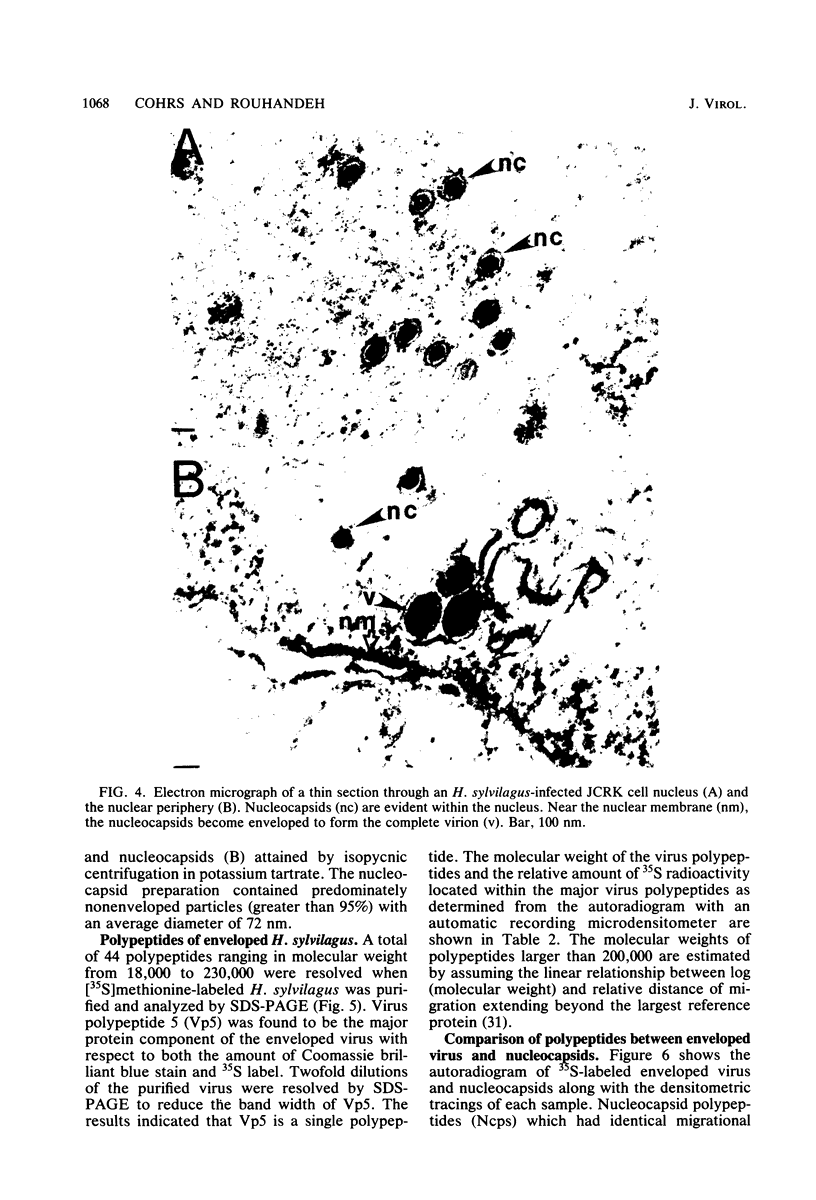
- Hinze H. C. New member of the herpesvirus group isolated from wild cottontail rabbits. Infect Immun. 1971 Feb;3(2):350–354. doi: 10.1128/iai.3.2.350-354.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H. C., Wegner D. L. Oncogenicity of rabbit herpesvirus. Cancer Res. 1973 Jun;33(6):1434–1435. [PubMed] [Google Scholar]
- Kemp M. C., Perdue M. L., Rogers H. W., O'Callaghan D. J., Randall C. C. Structural polypeptides of the hamster strain of equine herpes virus type 1: products associated with purification. Virology. 1974 Oct;61(2):361–375. doi: 10.1016/0042-6822(74)90274-8. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural proteins of purified murine cytomegalovirus. J Virol. 1976 Mar;17(3):906–915. doi: 10.1128/jvi.17.3.906-915.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ley K. D., Burger D. Cell-associated nature of cottontail rabbit herpesvirus in vitro. Appl Microbiol. 1970 Mar;19(3):549–550. doi: 10.1128/am.19.3.549-550.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Shemer Y., Leventon-Kriss S., Sarov I. Isolation and polypeptide characterization of varicella-zoster virus. Virology. 1980 Oct 15;106(1):133–140. doi: 10.1016/0042-6822(80)90228-7. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S. Virus-induced proteins in pseudorabies-infected cells. II. Proteins of the virion and nucleocapsid. J Virol. 1975 Oct;16(4):944–950. doi: 10.1128/jvi.16.4.944-950.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]