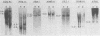Abstract
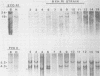
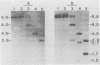
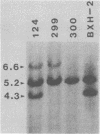
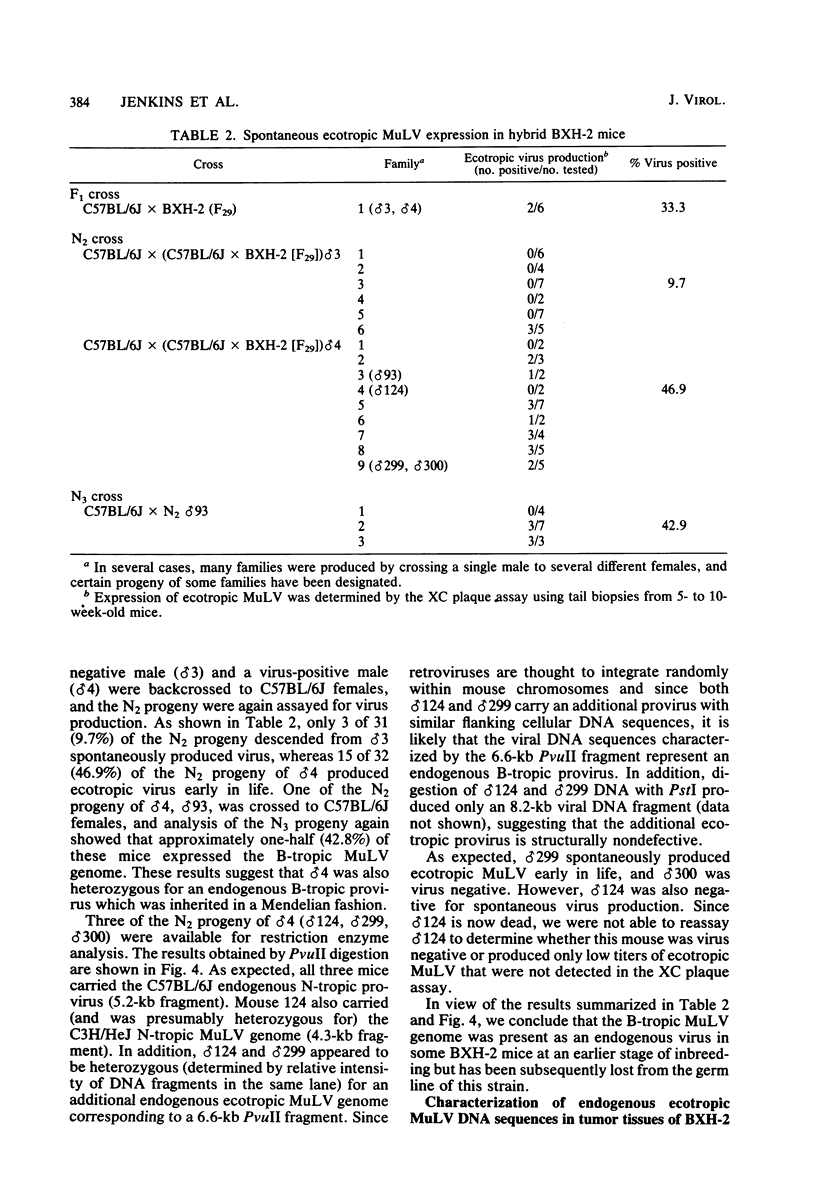
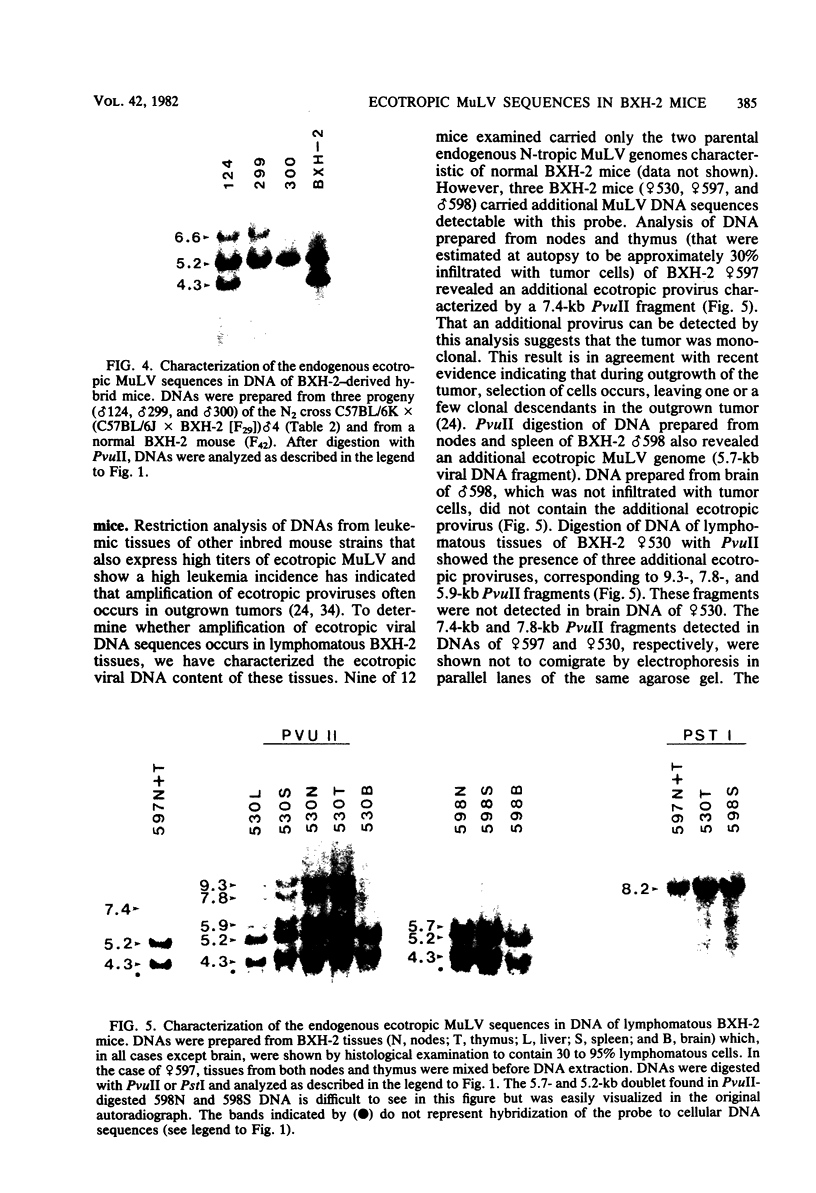
BXH-2 recombinant inbred mice spontaneously produce a B-tropic murine leukemia virus (MuLV) beginning early in life and have a high incidence of non-T-cell lymphomas. These traits are not characteristic of the progenitor strains (C57BL/6J and C3H/HeJ) or of 11 other BXH recombinant inbred strains. Since B-tropic virus expression may be causally related to the high incidence of lymphoma in this strain, we have analyzed the ecotropic MuLV DNA content of both normal and lymphomatous tissues of BXH-2 mice. Southern analysis and hybridization with an ecotropic MuLV DNA-specific probe showed that DNA of normal BXH-2 tissues contained both parental N-tropic MuLV proviruses but lacked endogenous B-tropic MuLV DNA sequences. In addition, none of 116 F1 hybrid mice derived from male BXH-2 mice spontaneously produced ecotropic MuLV early in life. These results suggest that the B-tropic virus is horizontally transmitted in BXH-2 mice. Southern analysis of DNA from tumor tissues of 12 BXH-2 mice showed that amplification of ecotropic-specific DNA sequences had occurred in lymphomatous tissues of 3 mice and suggested that these tumors were monoclonal. The number of newly acquired proviruses, which appeared to be structurally nondefective and integrated at different sites, varied from one to three copies. Since lymphomatous tissues from only 3 of 12 mice examined carried additional detectable ecotropic proviruses, these results suggest that amplification of ecotropic MuLV DNA sequences is not required for maintenance of transformation in BXH-2 lymphomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedigian H. G., Taylor B. A., Meier H. Expression of murine leukemia viruses in the highly lymphomatous BXH-2 recombinant inbred mouse strain. J Virol. 1981 Aug;39(2):632–640. doi: 10.1128/jvi.39.2.632-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benade L. E., Ihle J. N., Declève A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombatti A., Dux A., Berns A., Démant P., Hilgers J. H-2-dependent regulation of the high level of expression of ecotropic murine leukemia virus. J Natl Cancer Inst. 1979 Sep;63(3):869–873. doi: 10.1093/jnci/63.3.869. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Jensen F. C., Lerner R. A. In vitro construction of a B-tropic virus by recombination: B-tropism is a cryptic phenotype of xenotropic murine retroviruses. Proc Natl Acad Sci U S A. 1980 May;77(5):2989–2993. doi: 10.1073/pnas.77.5.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N. Experimental models and conceptual approaches to studies of lymphomas and leukemia: etiology, biology, and control. Semin Hematol. 1978 Apr;15(2):95–115. [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R., Domotor J. J., Jr Genetic linkage of C3H/HeJ and BALB/c endogenous ecotropic C-type viruses to phosphoglucomutase-1 on chromosome 5. Science. 1979 Apr 6;204(4388):71–73. doi: 10.1126/science.219476. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., McEwan R., Bengali K. Radiation leukemia in C57BL/6 mice. I. Lack of serological evidence for the role of endogenous ecotropic viruses in pathogenesis. J Exp Med. 1976 Dec 1;144(6):1391–1405. doi: 10.1084/jem.144.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Retroviruses and embryogenesis: microinjection of Moloney leukemia virus into midgestation mouse embryos. Cell. 1980 Jan;19(1):181–188. doi: 10.1016/0092-8674(80)90399-2. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Jaenisch R. Conformation of free and of integrated Moloney leukemia virus proviral DNA in preleukemic and leukemic BALB/Mo mice. Virology. 1980 Feb;101(1):111–123. doi: 10.1016/0042-6822(80)90488-2. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic type C viruses. Curr Top Microbiol Immunol. 1978;79:111–213. doi: 10.1007/978-3-642-66853-1_4. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Hartley J. W., Rowe W. P. Induction of B-tropic and N-tropic murine leukemia virus from B10.BR/SgLi mouse embryo cell lines by 5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1979 Jul;63(1):213–217. [PubMed] [Google Scholar]
- Morris V. L., Vlasschaert J. E., Beard C. L., Milazzo M. F., Bradbury W. C. Mammary tumors from BALB/c mice with a reported high mammary tumor incidence have acquired new mammary tumor virus DNA sequences. Virology. 1980 Jan 15;100(1):101–109. doi: 10.1016/0042-6822(80)90555-3. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Stephenson J. R., Cabradilla C. D., Aaronson S. A. Endogenous mouse type-C RNA virus of SWR cells: inducibility locus containing structural information for a new endogenous virus class. Virology. 1977 Oct 15;82(2):392–400. doi: 10.1016/0042-6822(77)90014-9. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephension J. R., Reynolds R. K., Tronick S. R., Aaronson S. A. Distribution of three classes of endogenous type-C RNA viruses among inbred strains of mice. Virology. 1975 Oct;67(2):404–414. doi: 10.1016/0042-6822(75)90442-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Induction of an endogenous B-tropic type C RNA virus from SWR/J mouse embryo cells in tissue culture. Virology. 1976 Apr;70(2):352–359. doi: 10.1016/0042-6822(76)90277-4. [DOI] [PubMed] [Google Scholar]
- Yoshimura F. K., Breda M. Lack of AKR ecotropic provirus amplification in AKR leukemic thymuses. J Virol. 1981 Sep;39(3):808–815. doi: 10.1128/jvi.39.3.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H., Quint W., van Raaij J., Maandag E. R., Verma I. M., Berns A. M-MuLV-induced leukemogenesis: integration and structure of recombinant proviruses in tumors. Cell. 1981 Jun;24(3):729–739. doi: 10.1016/0092-8674(81)90099-4. [DOI] [PubMed] [Google Scholar]
- van der Putten H., Terwindt E., Berns A., Jaenisch R. The integration sites of endogenous and exogenous Moloney murine leukemia virus. Cell. 1979 Sep;18(1):109–116. doi: 10.1016/0092-8674(79)90359-3. [DOI] [PubMed] [Google Scholar]