Abstract
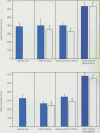
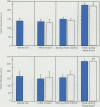
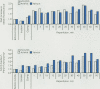
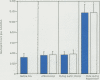
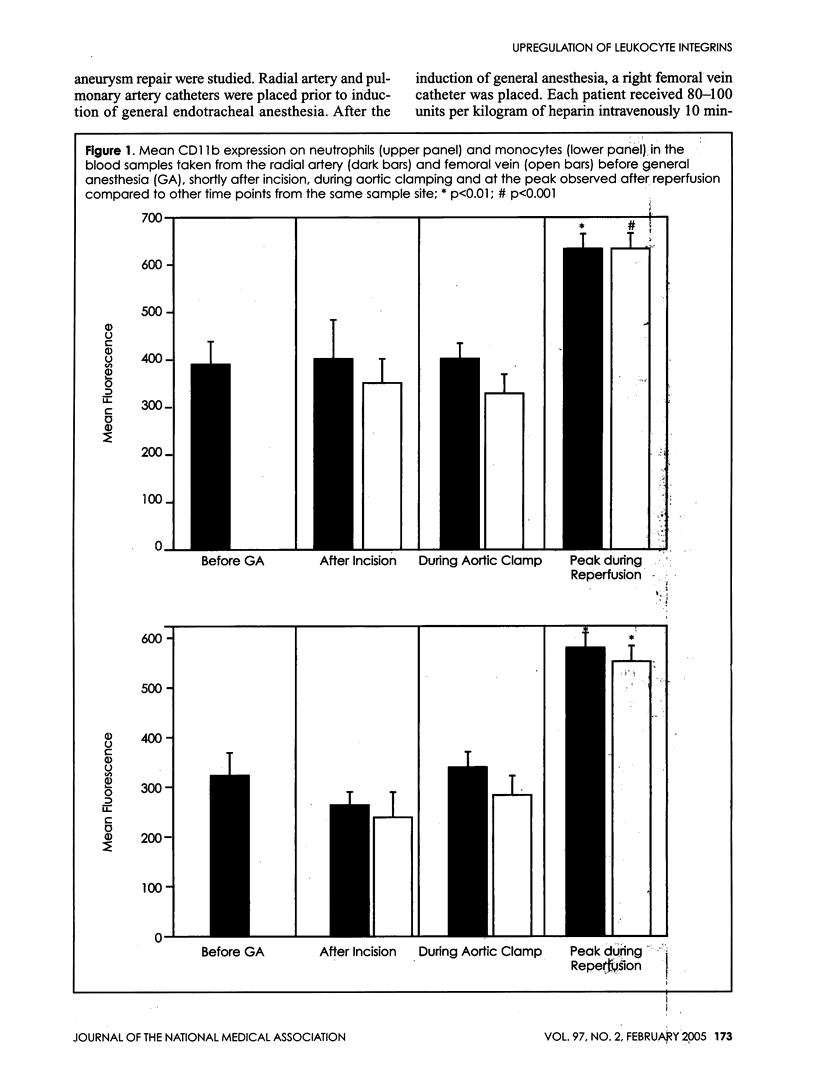
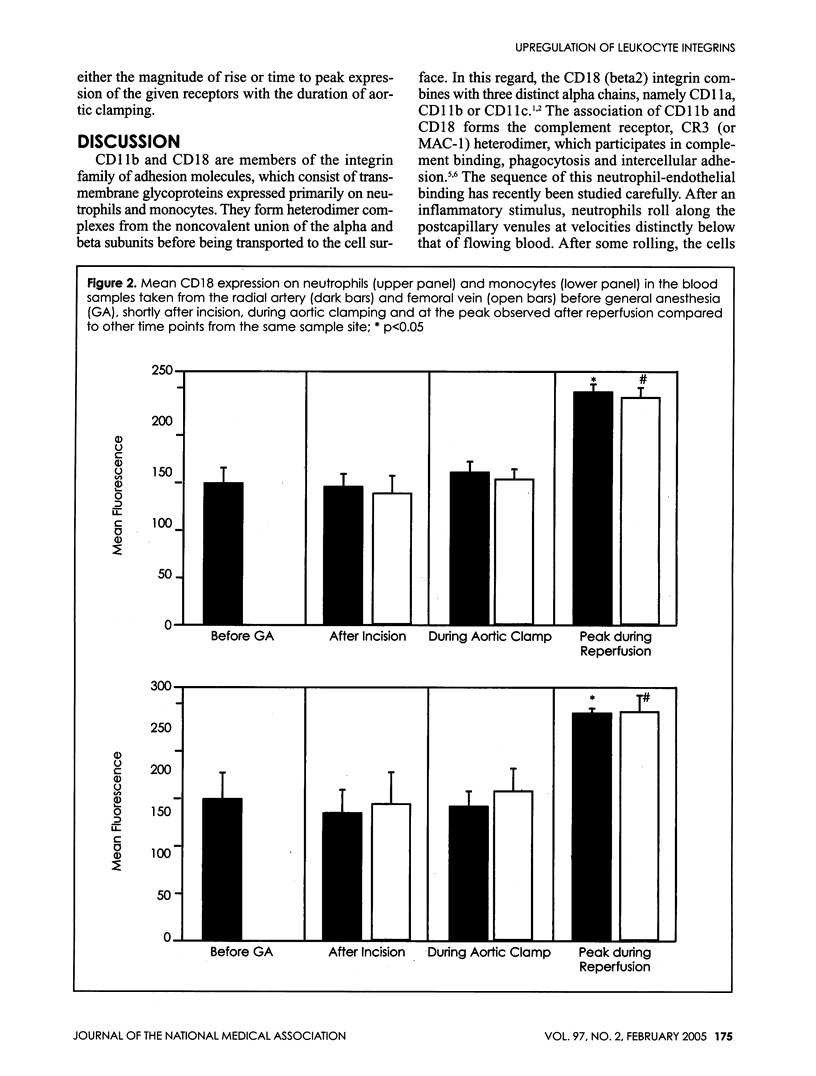
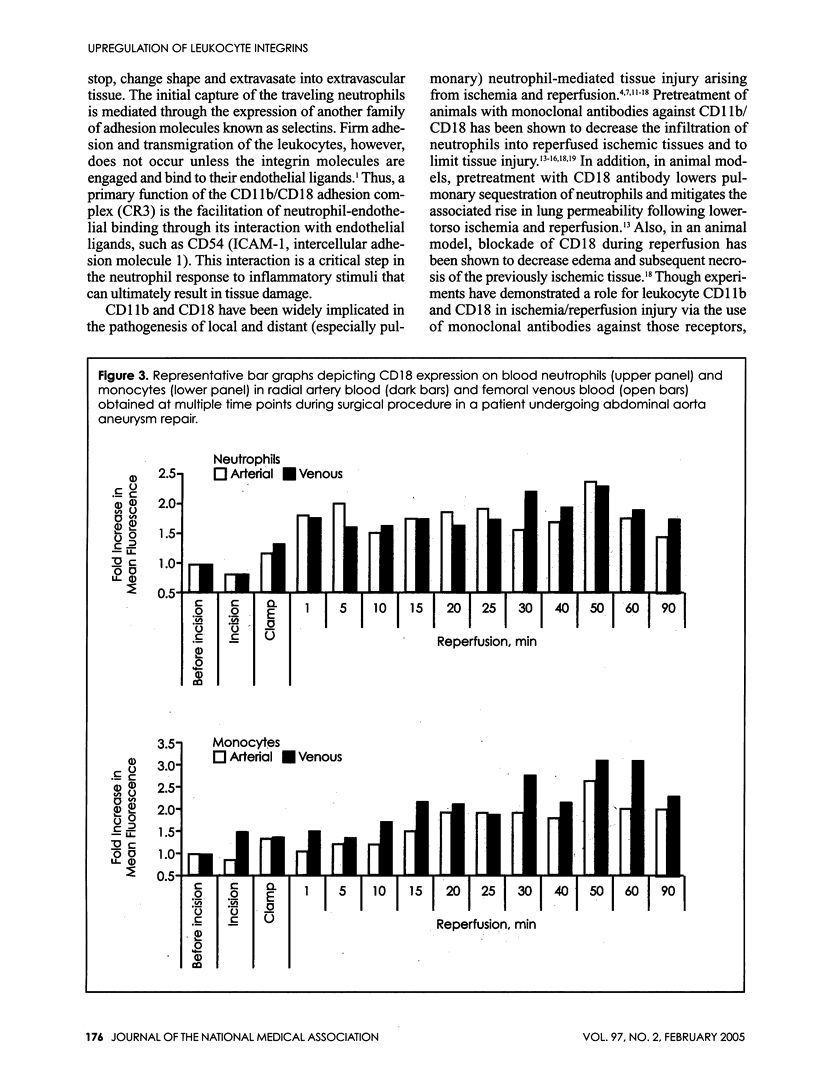
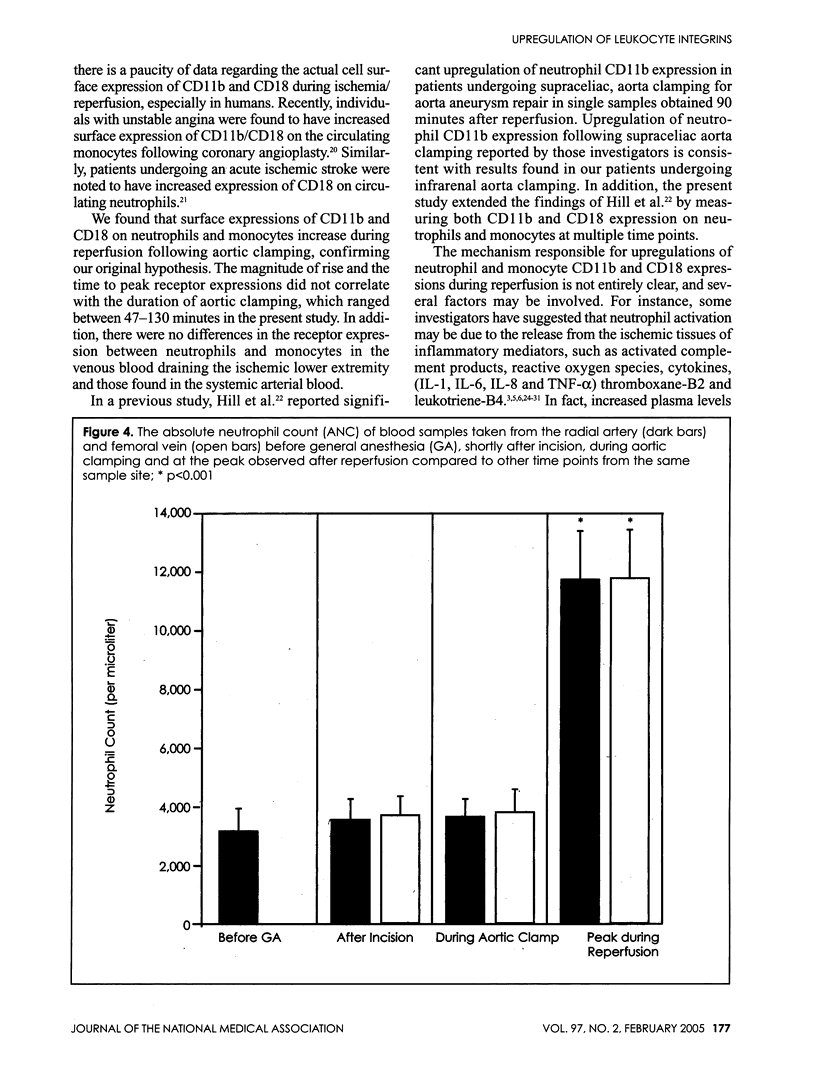
Ischemia and reperfusion in myocardial infarction and stroke are associated with upregulation of leukocyte adhesion molecules, which contributes to tissue injury by facilitating leukocyte adhesion and infiltration in the affected tissues. Surgical repair of the abdominal aortic aneurysm involves clamping and declamping of the aorta, which necessarily results in ischemia and reperfusion of the lower half of the body. Given the large volume of the affected tissues and unimpeded venous return during reperfusion, we hypothesized that the procedure may result in upregulation of leukocyte integrins in the systemic circulation. To test this hypothesis, we studied neutrophil and monocyte surface densities of CD11b and CD18 in patients undergoing elective infrarenal abdominal aortic aneurysm repair. Serial blood samples were collected from the radial artery and femoral vein during the operation and leukocyte CD11b and CD18 surface densities were quantified by flow cytometry. Following reperfusion, CD11b expression in neutrophils and monocytes increased significantly in femoral venous and arterial blood. The mean time to peak expression of CD11 b in neutrophils and monocytes during reperfusion was 34.4 and 31.4 minutes in venous and 38.5 and 36.4 minutes in arterial blood, respectively. Similar rises in CD18 expression on neutrophils and monocytes were observed in venous and arterial blood. The mean time to peak expression of CD18 in neutrophils and monocytes during reperfusion was 34.0 and 40.0 minutes in venous and 47.5 and 50.0 minutes in arterial blood, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai M., Lefer D. J., So T., DiPaula A., Aversano T., Becker L. C. An anti-CD18 antibody limits infarct size and preserves left ventricular function in dogs with ischemia and 48-hour reperfusion. J Am Coll Cardiol. 1996 Apr;27(5):1278–1285. doi: 10.1016/0735-1097(95)00578-1. [DOI] [PubMed] [Google Scholar]
- Benton L. D., Khan M., Greco R. S. Integrins, adhesion molecules and surgical research. Surg Gynecol Obstet. 1993 Sep;177(3):311–327. [PubMed] [Google Scholar]
- Caimi G., Canino B., Ferrara F., Montana M., Musso M., Porretto F., Carollo C., Catania A., Lo Presti R. Granulocyte integrins before and after activation in acute ischaemic stroke. J Neurol Sci. 2001 May 1;186(1-2):23–26. doi: 10.1016/s0022-510x(01)00495-6. [DOI] [PubMed] [Google Scholar]
- Cambria R. A., Anderson R. J., Dikdan G., Lysz T. W., Hobson RW I. I. Thromboxane synthetase inhibition decreases polymorphonuclear leukocyte activation following hindlimb ischemia. Am Surg. 1991 Feb;57(2):76–79. [PubMed] [Google Scholar]
- Cambria R. A., Anderson R. J., Dikdan G., Lysz T. W., Hobson R. W., 2nd The influence of arachidonic acid metabolites on leukocyte activation and skeletal muscle injury after ischemia and reperfusion. J Vasc Surg. 1991 Oct;14(4):549–556. [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993 Feb;36(2):147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- Frangogiannis Nikolaos G., Smith C. Wayne, Entman Mark L. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002 Jan;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Frijns C. J. M., Kappelle L. J. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke. 2002 Aug;33(8):2115–2122. doi: 10.1161/01.str.0000021902.33129.69. [DOI] [PubMed] [Google Scholar]
- Gadaleta D., Fantini G. A., Silane M. F., Davis J. M. Neutrophil leukotriene generation and pulmonary dysfunction after abdominal aortic aneurysm repair. Surgery. 1994 Nov;116(5):847–852. [PubMed] [Google Scholar]
- Gute D. C., Ishida T., Yarimizu K., Korthuis R. J. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Mol Cell Biochem. 1998 Feb;179(1-2):169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- Hill G. E., Mihalakakos P. J., Spurzem J. R., Baxter T. B. Supraceliac, but not infrarenal, aortic cross-clamping upregulates neutrophil integrin CD11b. J Cardiothorac Vasc Anesth. 1995 Oct;9(5):515–518. doi: 10.1016/s1053-0770(05)80133-8. [DOI] [PubMed] [Google Scholar]
- Hill J., Lindsay T., Rusche J., Valeri C. R., Shepro D., Hechtman H. B. A Mac-1 antibody reduces liver and lung injury but not neutrophil sequestration after intestinal ischemia-reperfusion. Surgery. 1992 Aug;112(2):166–172. [PubMed] [Google Scholar]
- Jerome S. N., Kong L., Korthuis R. J. Microvascular dysfunction in postischemic skeletal muscle. J Invest Surg. 1994 Jan-Feb;7(1):3–16. doi: 10.3109/08941939409018278. [DOI] [PubMed] [Google Scholar]
- Kerrigan C. L., Stotland M. A. Ischemia reperfusion injury: a review. Microsurgery. 1993;14(3):165–175. doi: 10.1002/micr.1920140307. [DOI] [PubMed] [Google Scholar]
- Klausner J. M., Paterson I. S., Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Leukotrienes but not complement mediate limb ischemia-induced lung injury. Ann Surg. 1989 Apr;209(4):462–470. doi: 10.1097/00000658-198904000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S., Ogawa S., Kuwabara K., Brett J., Leavy J. A., Ryan J., Koga Y., Plocinski J., Benjamin W., Burns D. K. Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J Clin Invest. 1992 Sep;90(3):1007–1015. doi: 10.1172/JCI115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metinko A. P., Kunkel S. L., Standiford T. J., Strieter R. M. Anoxia-hyperoxia induces monocyte-derived interleukin-8. J Clin Invest. 1992 Sep;90(3):791–798. doi: 10.1172/JCI115953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileski W. J., Winn R. K., Vedder N. B., Pohlman T. H., Harlan J. M., Rice C. L. Inhibition of CD18-dependent neutrophil adherence reduces organ injury after hemorrhagic shock in primates. Surgery. 1990 Aug;108(2):206–212. [PubMed] [Google Scholar]
- Paterson I. S., Klausner J. M., Pugatch R., Allen P., Mannick J. A., Shepro D., Hechtman H. B. Noncardiogenic pulmonary edema after abdominal aortic aneurysm surgery. Ann Surg. 1989 Feb;209(2):231–236. doi: 10.1097/00000658-198902000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton M., Anderson G., Vetvicka V., Justus D. E., Ross G. D. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993 Jun 1;150(11):5104–5113. [PubMed] [Google Scholar]
- Petrasek P. F., Liauw S., Romaschin A. D., Walker P. M. Salvage of postischemic skeletal muscle by monoclonal antibody blockade of neutrophil adhesion molecule CD18. J Surg Res. 1994 Jan;56(1):5–12. doi: 10.1006/jsre.1994.1002. [DOI] [PubMed] [Google Scholar]
- Qi Xiaoyong, Peng Yingxin, Gu Jian, Li Shuren, Zheng Shiling, Zhang Jianqing, Wang Tianhong. Inflammatory cytokine release in patients with unstable angina after coronary angioplasty. Jpn Heart J. 2002 Mar;43(2):103–115. doi: 10.1536/jhj.43.103. [DOI] [PubMed] [Google Scholar]
- Raijmakers P. G., Groeneveld A. B., Rauwerda J. A., Schneider A. J., Teule G. J., Hack C. E., Thijs L. G. Transient increase in interleukin-8 and pulmonary microvascular permeability following aortic surgery. Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 1):698–705. doi: 10.1164/ajrccm/151.3_Pt_1.698. [DOI] [PubMed] [Google Scholar]
- Rubin B. B., Smith A., Liauw S., Isenman D., Romaschin A. D., Walker P. M. Complement activation and white cell sequestration in postischemic skeletal muscle. Am J Physiol. 1990 Aug;259(2 Pt 2):H525–H531. doi: 10.1152/ajpheart.1990.259.2.H525. [DOI] [PubMed] [Google Scholar]
- Seekamp A., Mulligan M. S., Till G. O., Smith C. W., Miyasaka M., Tamatani T., Todd R. F., 3rd, Ward P. A. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993 Aug;143(2):464–472. [PMC free article] [PubMed] [Google Scholar]
- Seekamp A., Warren J. S., Remick D. G., Till G. O., Ward P. A. Requirements for tumor necrosis factor-alpha and interleukin-1 in limb ischemia/reperfusion injury and associated lung injury. Am J Pathol. 1993 Aug;143(2):453–463. [PMC free article] [PubMed] [Google Scholar]
- Sharar S. R., Mihelcic D. D., Han K. T., Harlan J. M., Winn R. K. Ischemia reperfusion injury in the rabbit ear is reduced by both immediate and delayed CD18 leukocyte adherence blockade. J Immunol. 1994 Sep 1;153(5):2234–2238. [PubMed] [Google Scholar]
- Swartbol P., Norgren L., Pärsson H., Truedsson L. Endovascular abdominal aortic aneurysm repair induces significant alterations in surface adhesion molecule expression on donor white blood cells exposed to patient plasma. Eur J Vasc Endovasc Surg. 1997 Jul;14(1):48–59. doi: 10.1016/s1078-5884(97)80225-0. [DOI] [PubMed] [Google Scholar]
- Terstappen L. W., Meiners H., Loken M. R. A rapid sample preparation technique for flow cytometric analysis of immunofluorescence allowing absolute enumeration of cell subpopulations. J Immunol Methods. 1989 Sep 29;123(1):103–112. doi: 10.1016/0022-1759(89)90034-3. [DOI] [PubMed] [Google Scholar]
- Welbourn R., Goldman G., Kobzik L., Paterson I. S., Valeri C. R., Shepro D., Hechtman H. B. Role of neutrophil adherence receptors (CD 18) in lung permeability following lower torso ischemia. Circ Res. 1992 Jul;71(1):82–86. doi: 10.1161/01.res.71.1.82. [DOI] [PubMed] [Google Scholar]
- Welbourn R., Goldman G., Kobzik L., Paterson I., Valeri C. R., Shepro D., Hechtman H. B. Neutrophil adherence receptors (CD 18) in ischemia. Dissociation between quantitative cell surface expression and diapedesis mediated by leukotriene B4. J Immunol. 1990 Sep 15;145(6):1906–1911. [PubMed] [Google Scholar]
- Welbourn R., Goldman G., O'Riordain M., Lindsay T. F., Paterson I. S., Kobzik L., Valeri C. R., Shepro D., Hechtman H. B. Role for tumor necrosis factor as mediator of lung injury following lower torso ischemia. J Appl Physiol (1985) 1991 Jun;70(6):2645–2649. doi: 10.1152/jappl.1991.70.6.2645. [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Seko Y., Tamatani T., Miyasaka M., Yagita H., Okumura K., Nagai R., Yazaki Y. Expression of intercellular adhesion molecule-1 in rat heart with ischemia/reperfusion and limitation of infarct size by treatment with antibodies against cell adhesion molecules. Am J Pathol. 1993 Aug;143(2):410–418. [PMC free article] [PubMed] [Google Scholar]