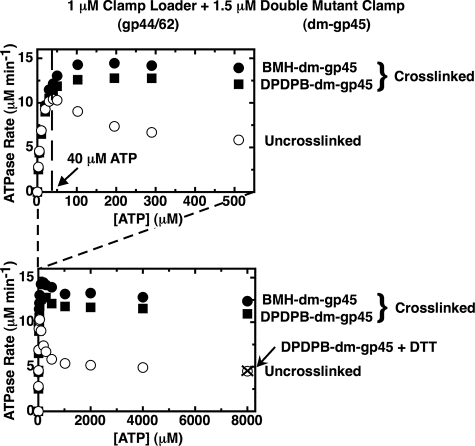
FIGURE 4.
Substrate inhibition is almost absent when the gp44/62 steady-state ATPase rates are measured in the presence of a covalently closed clamp. The steady-state ATPase rates of gp44/62 in the presence of uncross-linked double mutant gp45 (open symbols) and in the presence of cross-linked double mutant gp45 (closed symbols) cross-linked with either BMH (circles) or DPDPB (squares) were measured in the presence of an ATP regeneration system as described under “Experimental Procedures.” Component concentrations were 1 μm gp44/62 and 1.5 μm dm-gp45 uncross-linked (open symbols) or cross-linked (closed symbols), and the indicated concentrations of ATP. Also shown is the ATPase rate measured in the presence of 1 μm gp44/62 and 1.5 μm DPDPB-dm-gp45 after the covalent cross-links had been cleaved by incubation overnight with 10 mm DTT (× symbol).