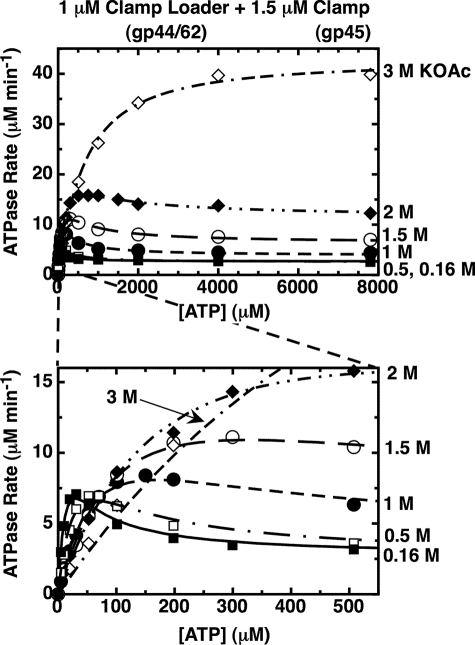
FIGURE 5.
The substrate inhibition effect on the ATPase rate profile for gp44/62-WT-gp45 is abolished as the monovalent salt concentration is increased. Component concentrations were 1 μm gp44/62, 1.5 μm gp45, and the indicated concentrations of ATP. The concentration of KOAc in the reaction buffer was 0.16 m (closed squares), 0.5 m (open squares), 1 m (closed circles), 1.5 m (open circles), 2 m (closed diamonds), or 3 m (open diamonds). Steady-state ATPase rates were measured in the presence of an ATP regeneration system (“Experimental Procedures”).