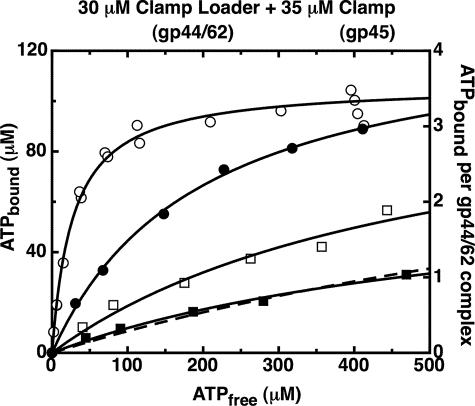
FIGURE 6.
The gp44/62-gp45 clamp loader-clamp complex binds four ATP molecules with similar affinities in salt concentrations up to 3 m KOAc. The ATP bound to gp44/62 under steady-state conditions in the presence of gp45 was measured using an ultrafiltration method as described under “Experimental Procedures.” Component concentrations were 30 μm gp44/62 and 35 μm WT-gp45. An ATP regeneration system was present in the reaction mixture. The concentration of KOAc in reaction buffer was 0.16 m (open circles), 1 m (closed circles), 2 m (open squares), and 3 m (closed squares). The data for 0.16 m KOAc (open circles) are the same as in Fig. 2. The curves were obtained by fitting the data points to a binding model (Equation 1) with four equal and independent ATP-binding sites, with Kd and the number of ATP sites per complex as floating parameters (continuous lines), or with Kd as the only floating parameter and the number of ATP sites per complex fixed at four (dashed line). The best fit values for Kd were 27 (±4) μm (0.16 m KOAc, open circles), 200 (±15) μm (1 m KOAc, closed circles), 500 (±240) μm (2 m KOAc, open squares), and 600 (±200) μm when both Kd and the number of ATP sites per complex in Equation 1 were allowed to float (3 m KOAc, closed squares, continuous line) or 1,300 (±60) μm when the number of ATP sites per complex in Equation 1 was fixed at four (3 m KOAc, closed squares, dotted line). The best fit values for the number of ATP sites per complex were 3.6 (±0.1) (0.16 m KOAc, open circles), 4.4 (±0.2) (1 m KOAc, closed circles), 3.7 (±1) (2 m KOAc, open squares), and 2.3 (±0.5) when both Kd and the number of ATP sites per complex in Equation 1 were allowed to float (3 m KOAc, closed squares, continuous line).