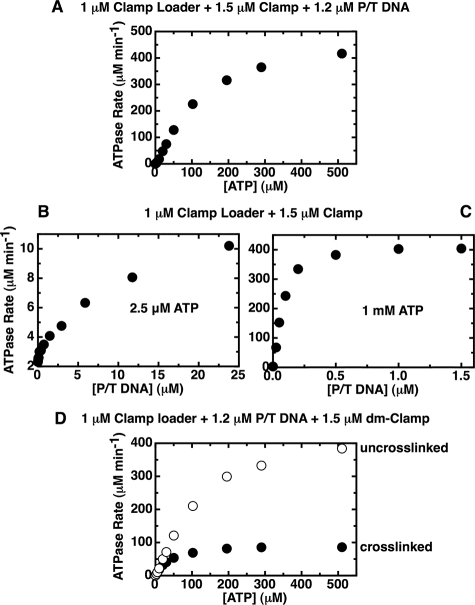
FIGURE 8.
The DNA-stimulated gp44/62 ATPase rates suggest that the ring opens up as more ATP is added. Steady-state ATPase rates were measured in the presence of an ATP regeneration system, as described under “Experimental Procedures.” A, gp44/62 ATPase activity measured as a function of ATP in the presence of gp45 and P/T DNA cofactors displays a sigmoidal rate profile. Component concentrations were 1 μm gp44/62, 1.5 μm gp45, 1.2 μm P/T DNA, and the indicated amounts of ATP. B and C, binding of P/T DNA to the gp44/62-gp45 complex is slow at low ATP concentrations and fast at high ATP concentrations. Component concentrations were 1 μm gp44/62, 1.5 μm gp45, and 2.5 μm (B) or 1 mm (C) ATP, plus the indicated amount of P/T DNA. D, DNA-stimulated gp44/62 ATPase rates measured in the presence of an open and closed dm clamp. Component concentrations were 1 μm gp44/62, 1.2 μm P/T DNA, 1.5 μm uncross-linked dm-gp45 (open symbols), or BMH-cross-linked dm-gp45 (closed symbols), and the indicated amount of ATP.