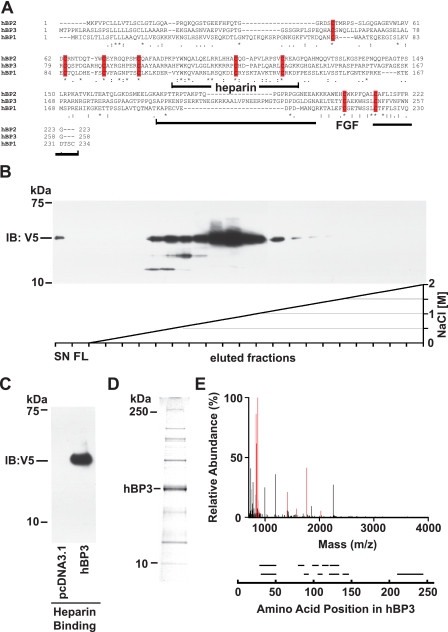
FIGURE 1.
Human BP3 sequence comparison, protein purification, and protein identification. A, amino acid sequence alignment of human BP1, -2, and -3 proteins (GenBank™: NP_005121, NP_114156, and NP_689642, respectively). The BP protein family conserved domain is PFAM_06473. Identical (*) and conserved (: or.) amino acids as well as conserved cysteine residues (red bars) are indicated. The heparin binding and the C-terminal FGF binding domains in BP1 (41) as well as the corresponding portions of BP3 are indicated. B and C, heparin binding of hBP3 harvested from conditioned media of transfected HEK293T cells. Heparin affinity chromatography on HiTrap FPLC colums using an NaCl gradient as indicated (C) and batch elution from heparin-Sepharose with SDS-PAGE loading buffer (D) are shown. Supernatants from cells transfected with a hBP3-V5/His expression vector (for 72 h) were loaded onto column that contains immobilized heparin as an affinity matrix. Eluted hBP3 was detected by immunoblotting (IB) for V5. SN, supernatant; FL, flow. D, Coomassie Blue staining of proteins in the peak elution fractions of hBP3 in panel B. The indicated band matched with the migration of immunoreactive hBP3 and was excised for mass spectrometry analysis. E, mass spectrometry analysis identified eleven peptides matching with hBP3 by peptide mass fingerprinting (in red). Peptide fragments identified by sequencing are shown aligned with their positions in the hBP3 protein.