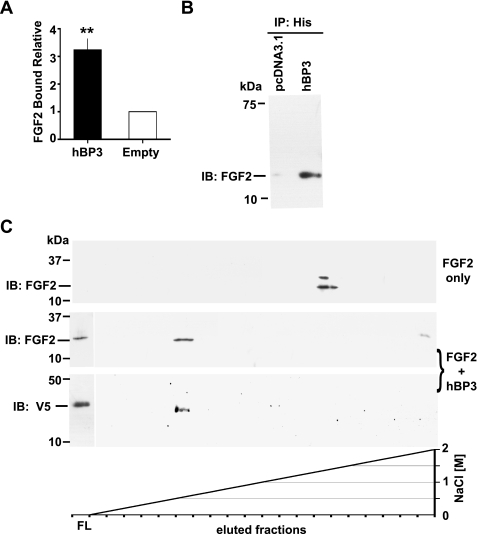
FIGURE 2.
hBP3 interaction with FGF2 and heparin. A, FGF2 binding to immobilized hBP3. hBP3 purified by heparin affinity (see Fig. 1D) or control media were immobilized in 96-well plates and then incubated with FGF2. After washing, bound FGF2 was detected by a mouse monoclonal antibody and horseradish peroxidase-conjugated goat anti-mouse secondary antibody. Binding relative to control is shown (mean ± S.E.; **, p < 0.01). B, interaction of hBP3 with endogenous FGF2. Anti-His antibody immunoprecipitation (IP) of proteins in supernatants from hBP3-V5/His or empty vector (pcDNA3.1) transfected 293T cells were immunoblotted for FGF2 to detect the interaction of hBP3 with endogenous FGF2. C, impact of hBP3 on FGF2 binding to heparin. FGF2 (1 μg) alone or a mixture of FGF2 plus an excess of hBP3 were loaded onto heparin affinity columns and bound proteins eluted by increasing concentrations of NaCl. FGF2 and hBP3 were detected by immunoblotting of aliquots. FGF2 only: in the absence of hBP3, FGF2 bound tightly to heparin and eluted at high salt concentrations (>1.5 m NaCl). FGF2 plus hBP3: As a complex, both proteins bound poorly to heparin and were mostly found in the flow (FL) from the column, although a smaller portion of FGF2 eluted with hBP3 at low salt concentrations (0.6 to 0.8 m NaCl).