Abstract
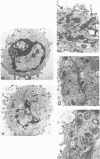
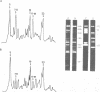
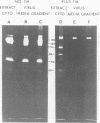
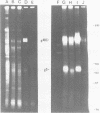
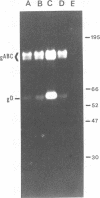
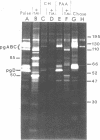
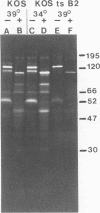
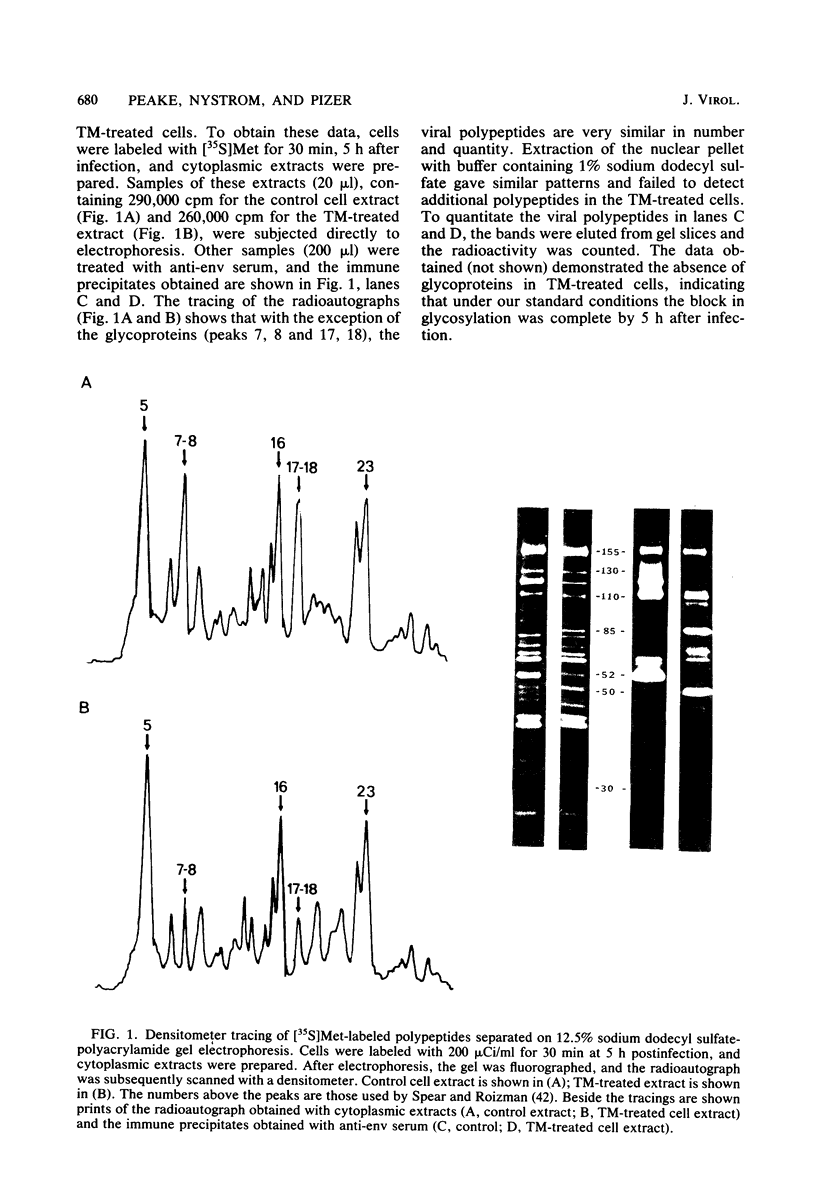
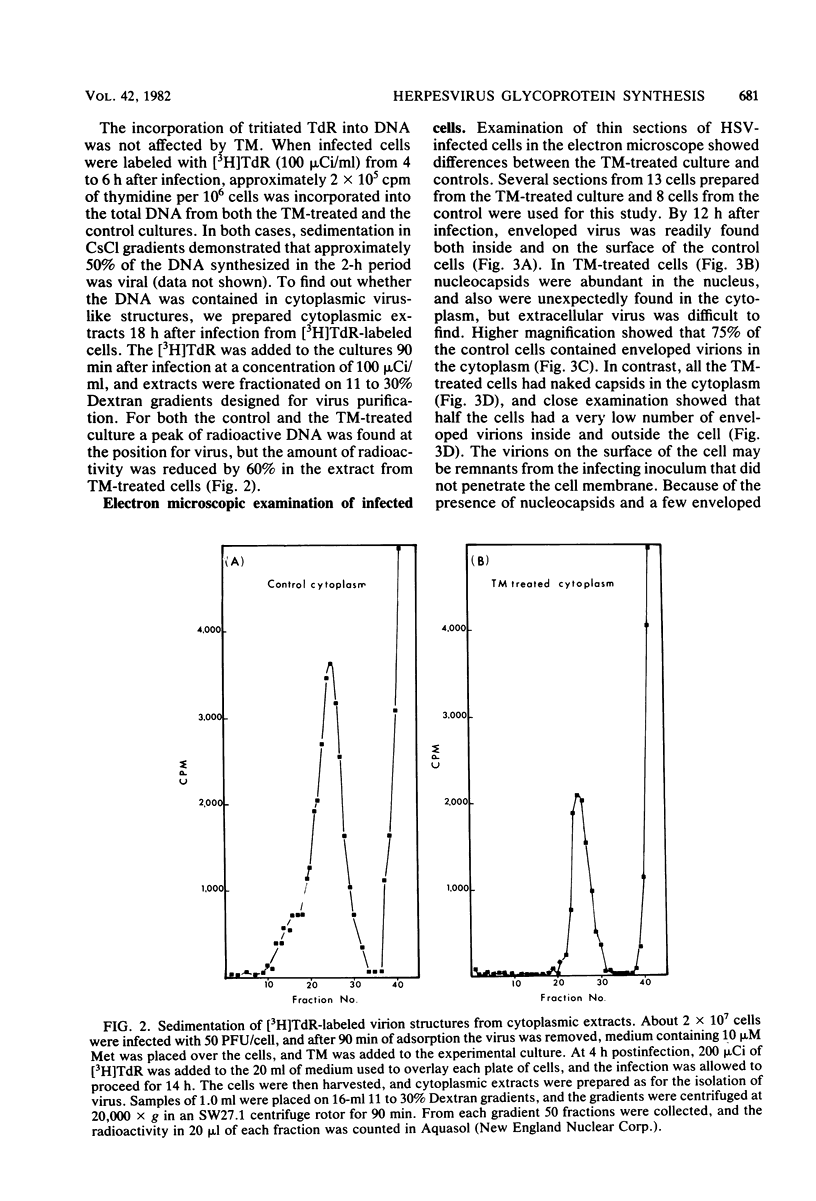
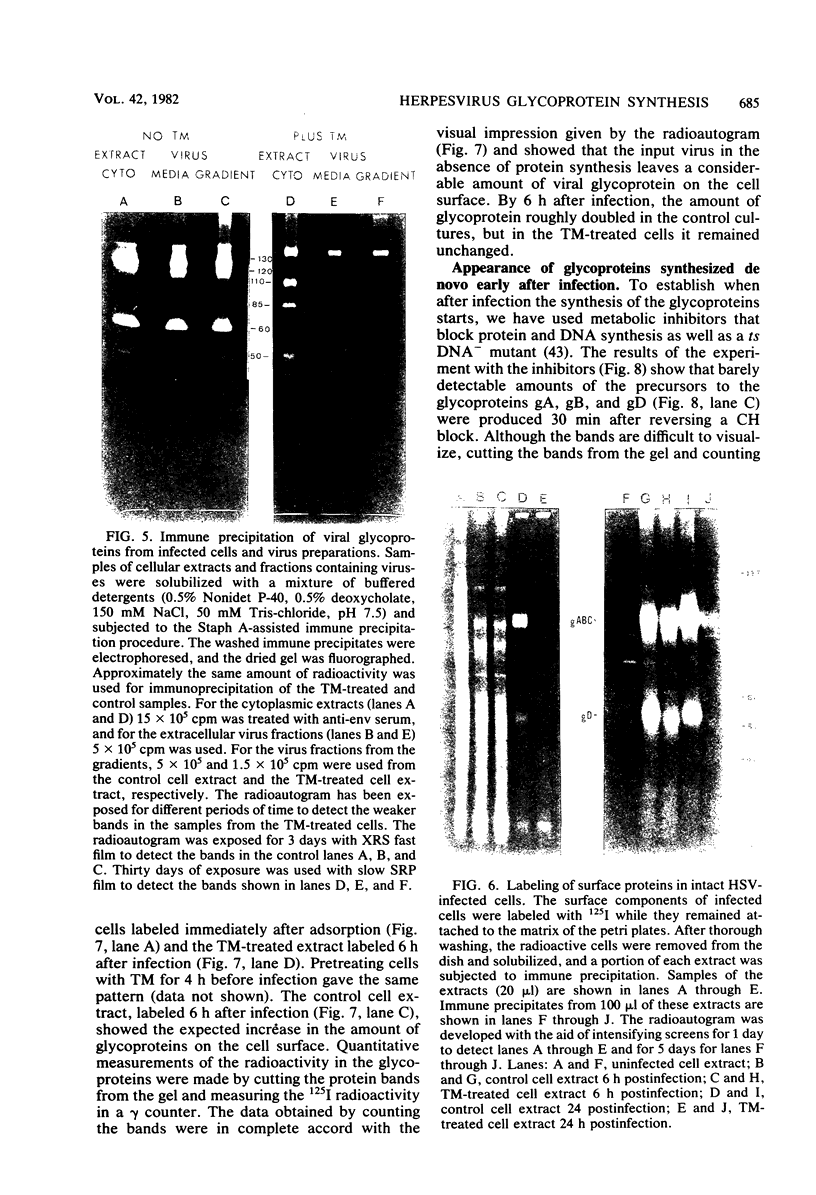
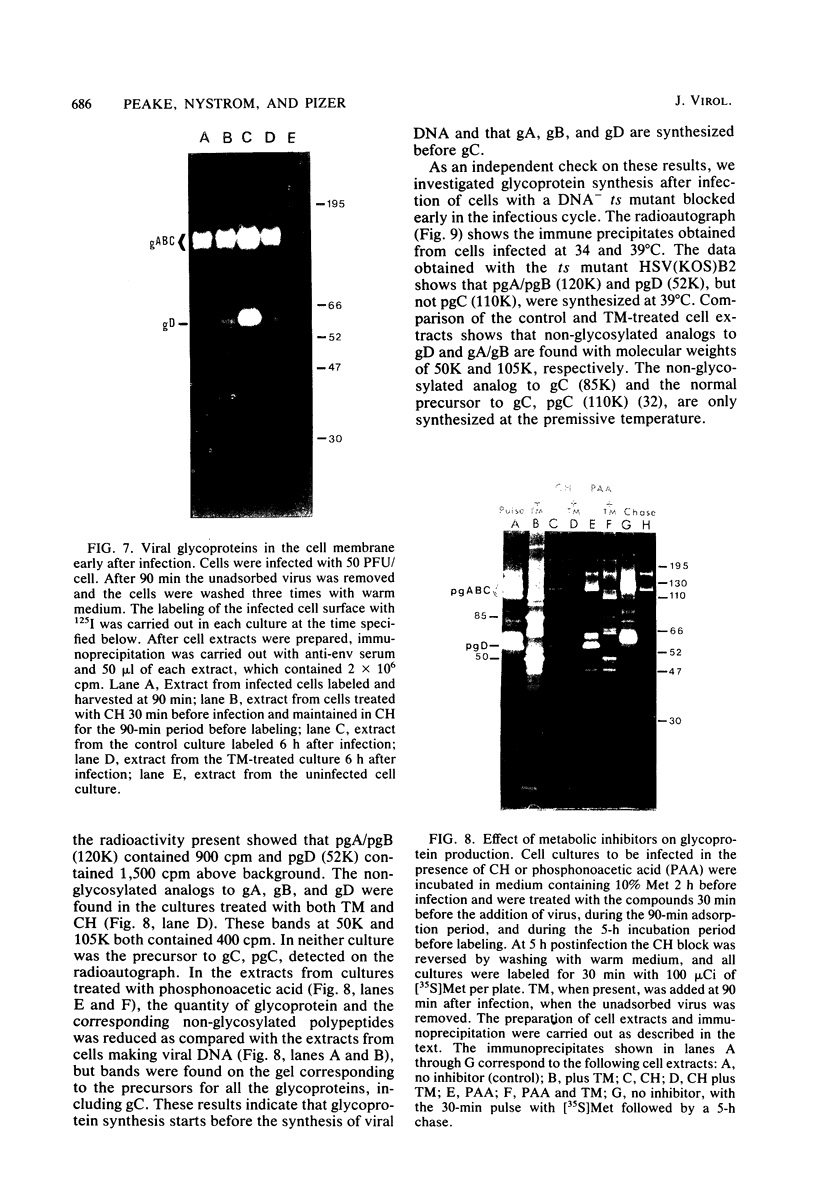
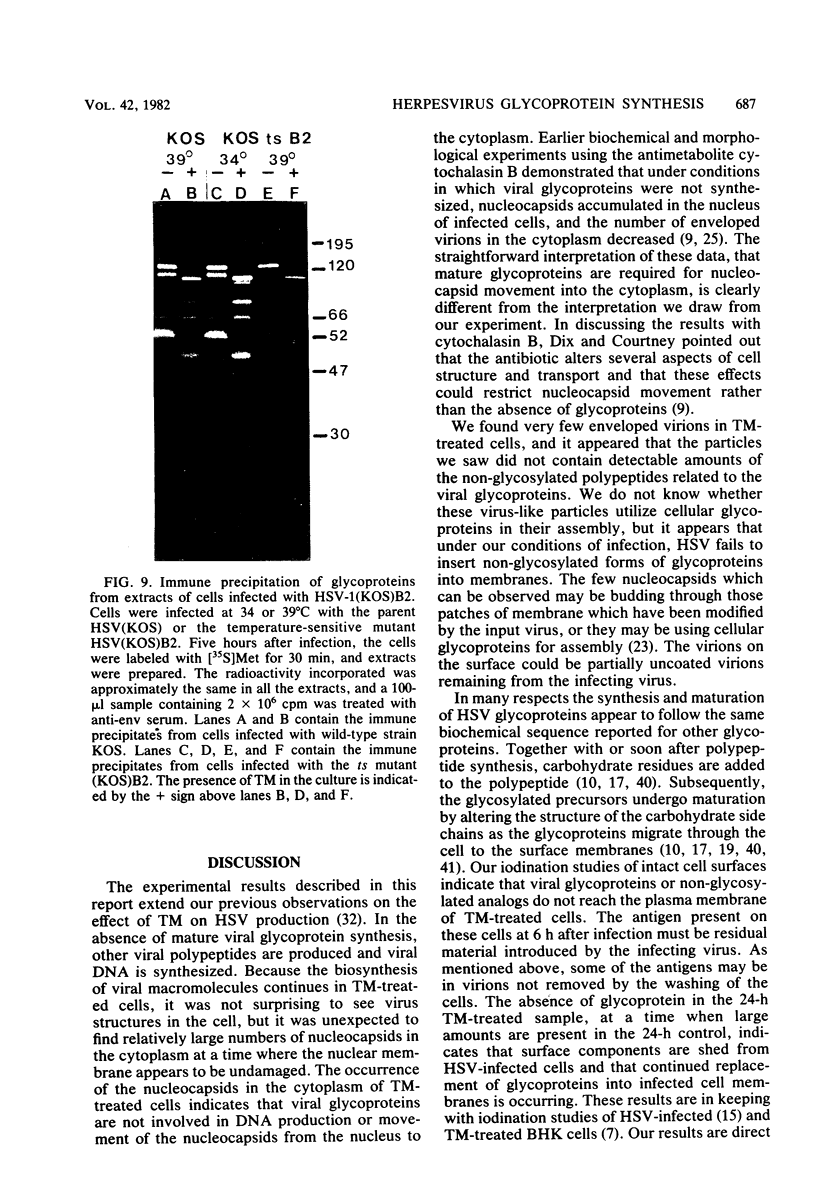
In the presence of the antibiotic tunicamycin (TM), glycosylation of herpes simplex virus glycoproteins is inhibited and non-glycosylated polypeptides analogous to the glycoproteins are synthesized (Pizer et al., J. Virol. 34:142-153, 1980). The synthesis of viral proteins and DNA occurs in TM-treated cells. By electron microscopy, nucleocapsids can be observed both in the nucleus and the cytoplasm of TM-treated cells; a small number of enveloped virions were observed on the cell surface. Analyses of the proteins in partially purified virus readily detects viral glycoproteins in the control cells, but neither glycoproteins nor nonglycosylated polypeptide analogs were observed in the virus prepared from TM-treated cells. By labeling the surface of infected cells with 125I, viral glycoproteins were detected as soon as 90 min after infection even when protein synthesis was inhibited with cycloheximide and glycosylation was blocked with TM. Labeling the proteins synthesized in infected cells with [35S]methionine showed that the surface glycoproteins detected in the cycloheximide- and TM-treated cells were not synthesized de novo after infection, but were placed on the cell surface by the infecting virus. Studies with metabolic inhibitors and a temperature-sensitive mutant blocked early in the infectious cycle showed that glycoproteins gA/gB and gD were synthesized soon after infection, but that the synthesis of gC was delayed. Under conditions of infection, in which gC and its precursor pgC are not produced, we have been able to observe the relationships between the glycosylated polypeptides that correspond to pgA/pgB and the nonglycosylated analog made in the presence of TM.
Full text
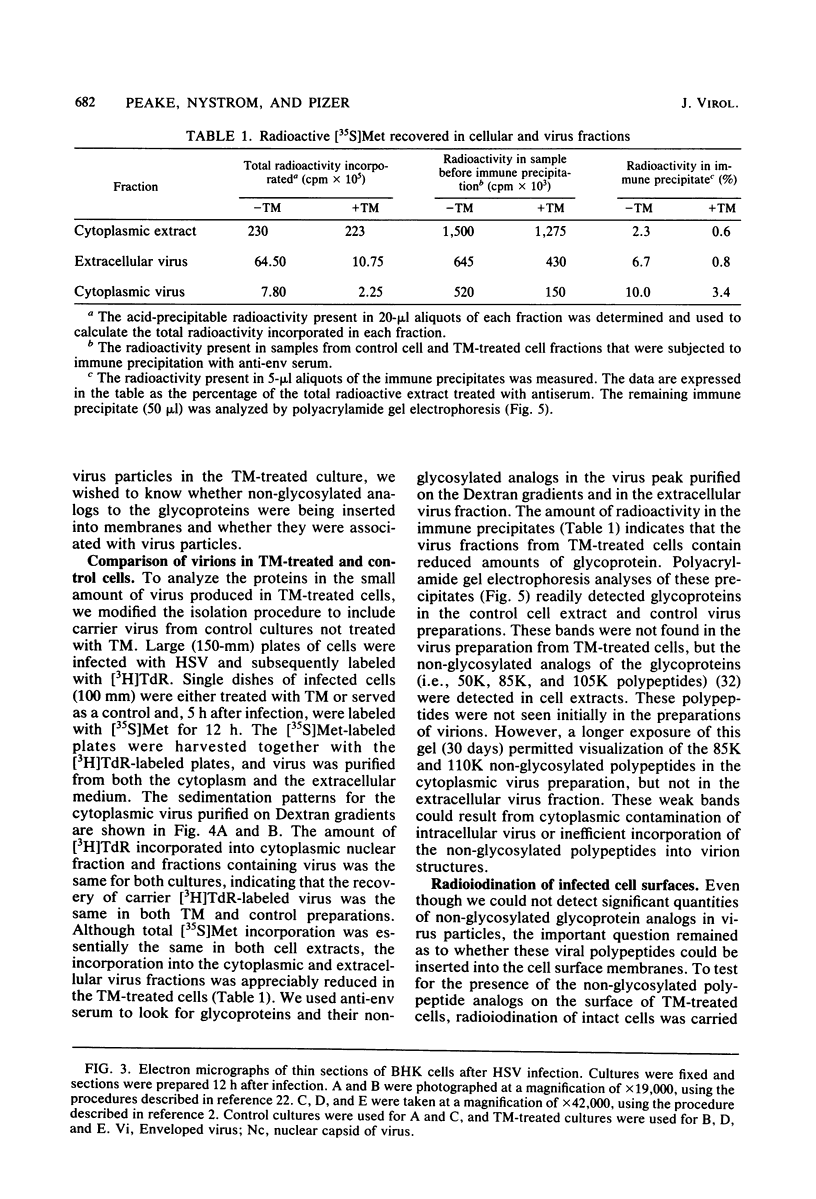
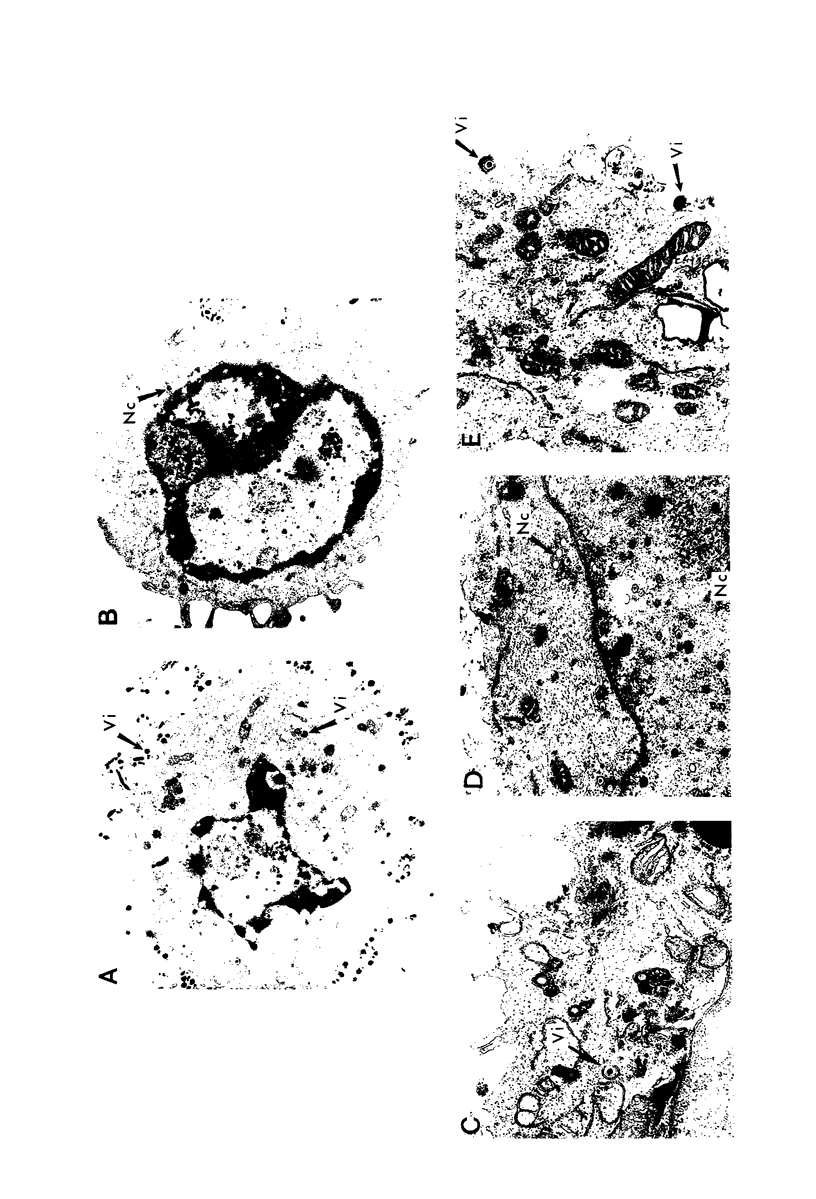
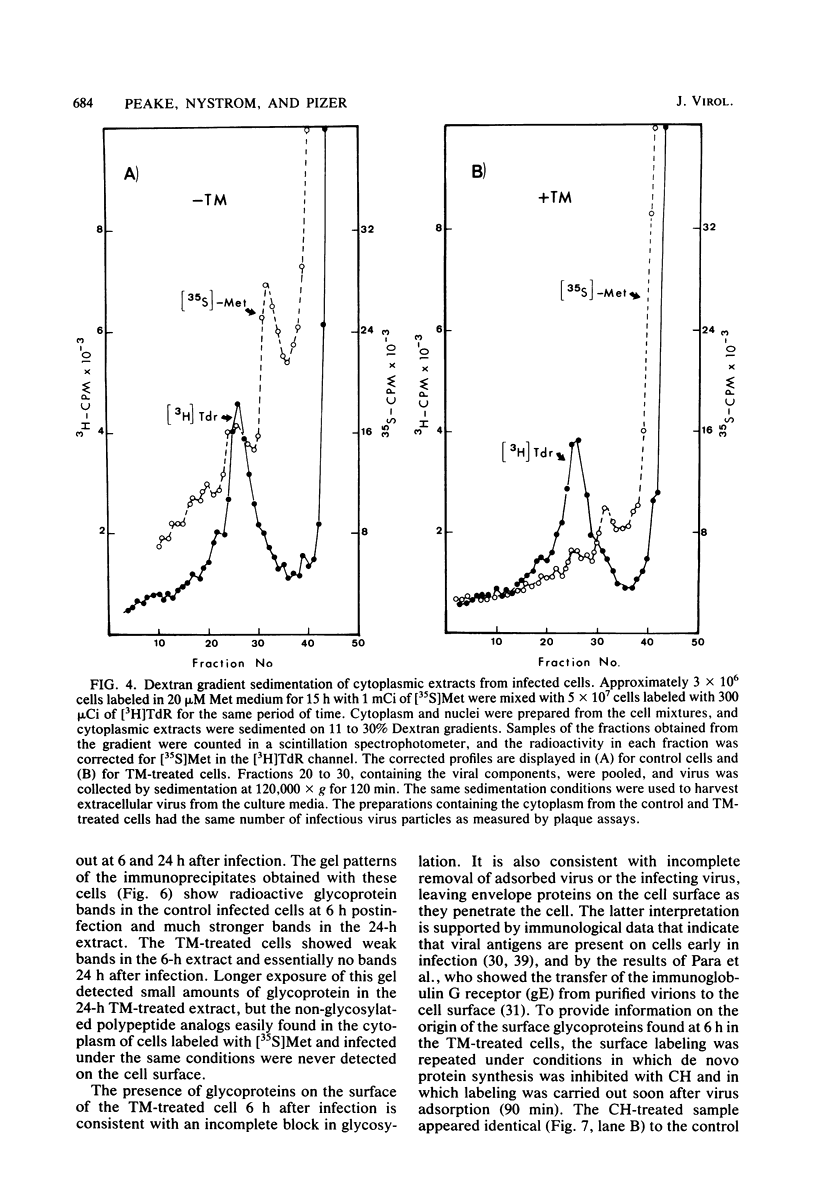
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter V. C., Schaffer P. A., Tevethia S. S. The involvement of herpes simplex virus type 1 glycoproteins in cell-mediated immunity. J Immunol. 1981 May;126(5):1655–1660. [PubMed] [Google Scholar]
- Chu C. T., Parris D. S., Dixon R. A., Farber F. E., Schaffer P. A. Hydroxylamine mutagenesis of HSV DNA and DNA fragments: introduction of mutations into selected regions of the viral genome. Virology. 1979 Oct 15;98(1):168–181. doi: 10.1016/0042-6822(79)90535-x. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Schaffer P. A., Powell K. L. Synthesis of virus-specific polypaptides by temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1976 Dec;75(2):306–318. doi: 10.1016/0042-6822(76)90030-1. [DOI] [PubMed] [Google Scholar]
- Cromeans T. L., Shore S. L. Lysis of herpes simplex virus-infected cells early in the infectious cycle by human antiviral antibody and complement. Infect Immun. 1981 Mar;31(3):1054–1061. doi: 10.1128/iai.31.3.1054-1061.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Levy-Benshimol A., Buck C. A., Warren L. Effect of tunicamycin on the synthesis, intracellular transport and shedding of membrane glycoproteins in BHK cells. Exp Cell Res. 1979 Mar 1;119(1):1–13. doi: 10.1016/0014-4827(79)90329-x. [DOI] [PubMed] [Google Scholar]
- Diggelmann H. Biosynthesis of an unglycosylated envelope glycoprotein of Rous sarcoma virus in the presence of tunicamycin. J Virol. 1979 Jun;30(3):799–804. doi: 10.1128/jvi.30.3.799-804.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., Courtney R. J. Effects of cytochalasin B on herpes simplex virus type 1 replication. Virology. 1976 Mar;70(1):127–135. doi: 10.1016/0042-6822(76)90242-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Schwarz R. T. Glycosylation is not necessary for membrane insertion and cleavage of Semliki Forest virus membrane proteins. Nature. 1978 Aug 3;274(5670):487–490. doi: 10.1038/274487a0. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Glorioso J. C., Levine M., Holland T. C., Szczesiul M. S. Mutant analysis of herpes simplex virus-induced cell surface antigens: resistance to complement-mediated immune cytolysis. J Virol. 1980 Sep;35(3):672–681. doi: 10.1128/jvi.35.3.672-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Margalith E., Duksin D. Antiviral activity of tunicamycin on herpes simplex virus. Antimicrob Agents Chemother. 1980 Jun;17(6):1014–1022. doi: 10.1128/aac.17.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. 3. Viruses differing in their effects on the social behavior of infected cells specify different membrane glycoproteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):865–871. doi: 10.1073/pnas.65.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Impaired intracellular migration and altered solubility of nonglycosylated glycoproteins of vesicular stomatitis virus and Sindbis virus. J Biol Chem. 1977 Dec 25;252(24):9018–9023. [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Wirth D., Porter M. Synthesis and assembly of viral membrane proteins. Ann N Y Acad Sci. 1980;343:319–337. doi: 10.1111/j.1749-6632.1980.tb47261.x. [DOI] [PubMed] [Google Scholar]
- Machtiger N. A., Pancake B. A., Eberle R., Courtney R. J., Tevethia S. S., Schaffer P. A. Herpes simplex virus glycoproteins: isolation of mutants resistant to immune cytolysis. J Virol. 1980 May;34(2):336–346. doi: 10.1128/jvi.34.2.336-346.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F., Dix R. D., Cabral G. A., Courtney R. J. Effects of cytochalasin B on the maturation of herpes simplex virus type 1: an ultrastructural investigation. Virology. 1977 Feb;76(2):860–865. doi: 10.1016/0042-6822(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Crombie I. K., Subak-Sharpe J. H. Control of protein synthesis in herpesvirus-infected cells: analysis of the polypeptides induced by wild type and sixteen temperature-sensitive mutants of HSV strain 17. J Gen Virol. 1976 Jun;31(3):347–372. doi: 10.1099/0022-1317-31-3-347. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Cromeans T. L., Nahmias A. J. Participation of three major glycoprotein antigens of herpes simplex virus type 1 early in the infectious cycle as determined by antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1980 Apr;28(1):38–44. doi: 10.1128/iai.28.1.38-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Kim S. U., Nystrom P., Coates V. C. Herpes simplex virus replication in pheochromocytoma cell line that responds to nerve growth factor. Acta Neuropathol. 1978 Oct 13;44(1):9–14. doi: 10.1007/BF00691633. [DOI] [PubMed] [Google Scholar]
- Preston V. G. Fine-structure mapping of herpes simplex virus type 1 temperature-sensitive mutations within the short repeat region of the genome. J Virol. 1981 Jul;39(1):150–161. doi: 10.1128/jvi.39.1.150-161.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Katz F. N., Lodish H. F. Glycosylation of a membrane protein is restricted to the growing polypeptide chain but is not necessary for insertion as a transmembrane protein. Cell. 1978 Dec;15(4):1447–1454. doi: 10.1016/0092-8674(78)90068-5. [DOI] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Levine M., Glorioso J. C. Method for induction of mutations in physically defined regions of the herpes simplex virus genome. J Virol. 1981 Apr;38(1):41–49. doi: 10.1128/jvi.38.1.41-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Rohrschneider J. M., Schmidt M. F. Suppression of glycoprotein formation of Semliki Forest, influenza, and avian sarcoma virus by tunicamycin. J Virol. 1976 Sep;19(3):782–791. doi: 10.1128/jvi.19.3.782-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Cromeans T. L., Norrild B. Early damage of herpes-infected cells by antibody-dependent cellular cytotoxicity: relative roles of virus-specified cell-surface antigens and input virus. J Immunol. 1979 Nov;123(5):2239–2244. [PubMed] [Google Scholar]
- Spear P. G., Kellejmroian B. Proteins spcified by herpes simplex virus. II. Viral glycoprotins associated with cellular membranes. J Virol. 1970 Feb;5(2):123–131. doi: 10.1128/jvi.5.2.123-131.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R., Spector D., Pizer L. Modulation of herpes simplex virus replication in adenovirus transformed cells. J Gen Virol. 1979 Aug;44(2):297–309. doi: 10.1099/0022-1317-44-2-297. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Lodish H. F., Robbins P. W. Requirements for the insertion of the Sindbis envelope glycoproteins into the endoplasmic reticulum membrane. J Cell Biol. 1979 Apr;81(1):154–162. doi: 10.1083/jcb.81.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]