Abstract
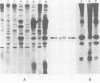
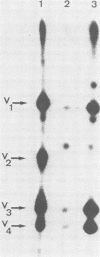
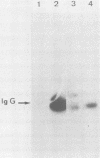
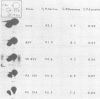
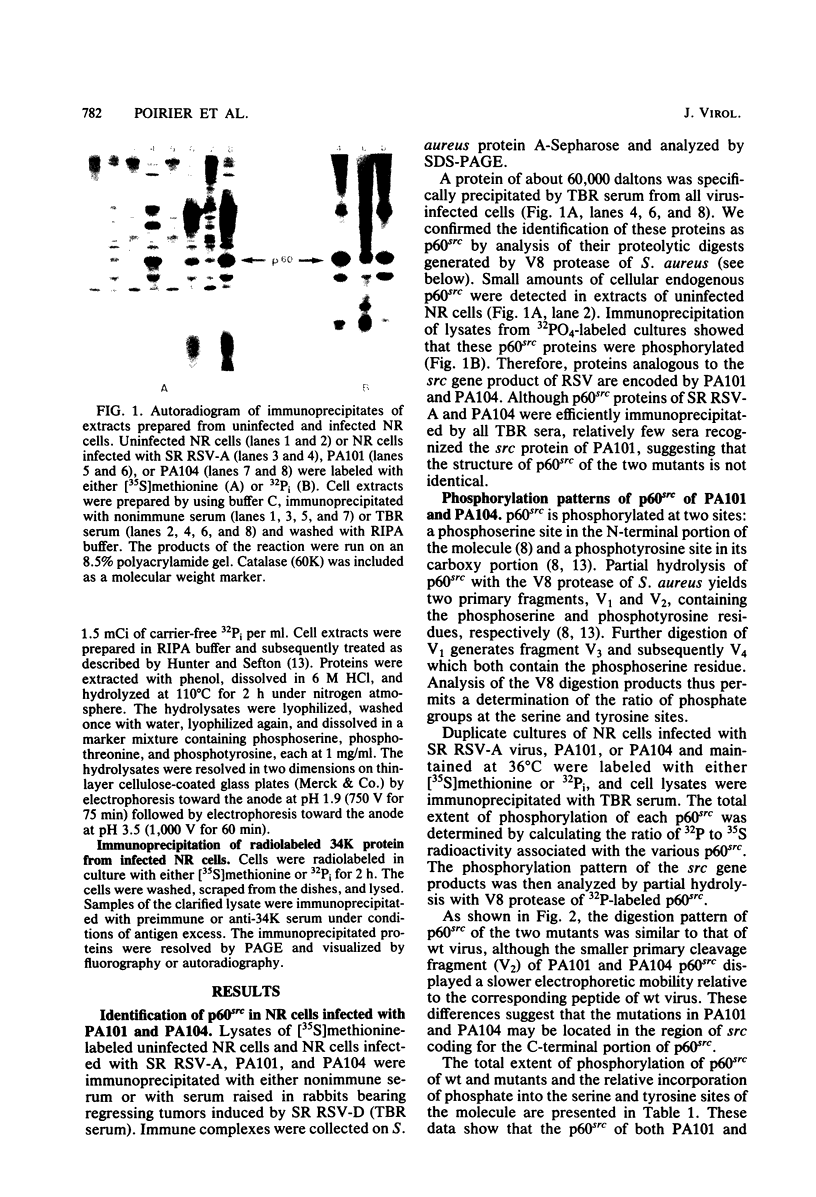
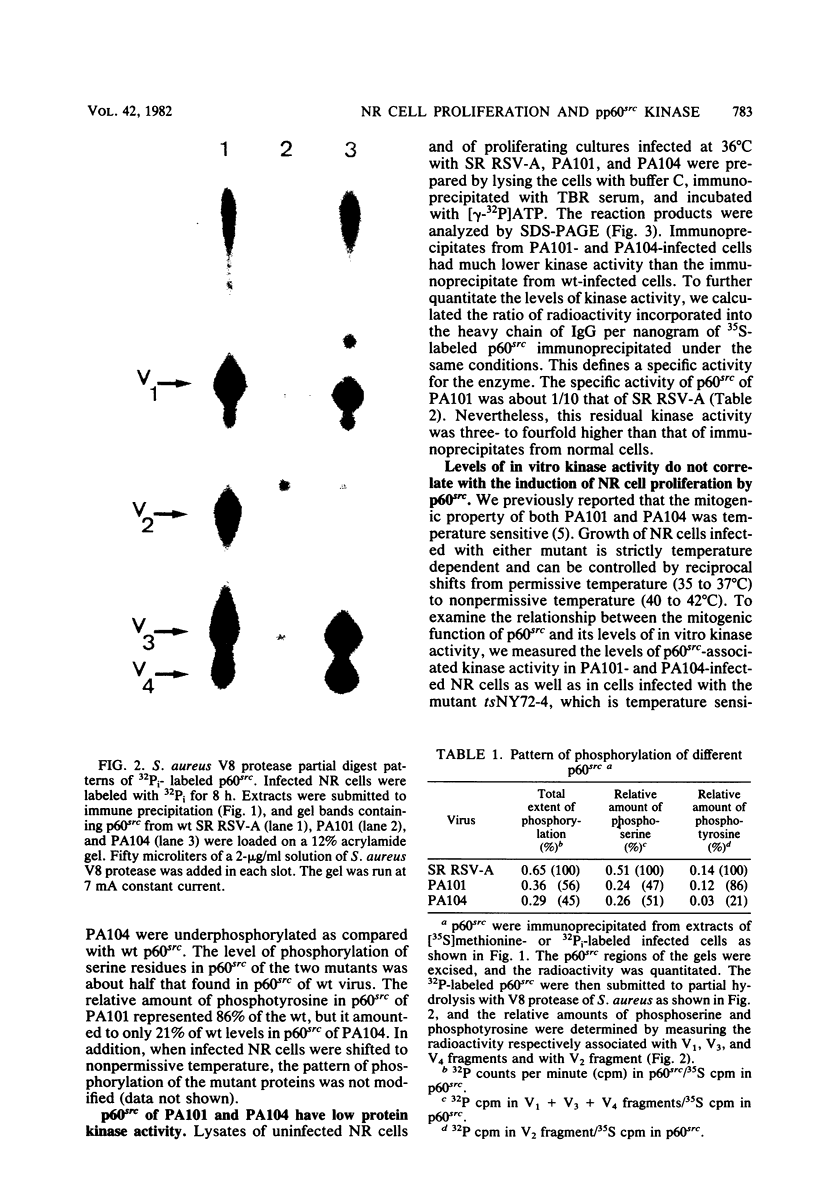
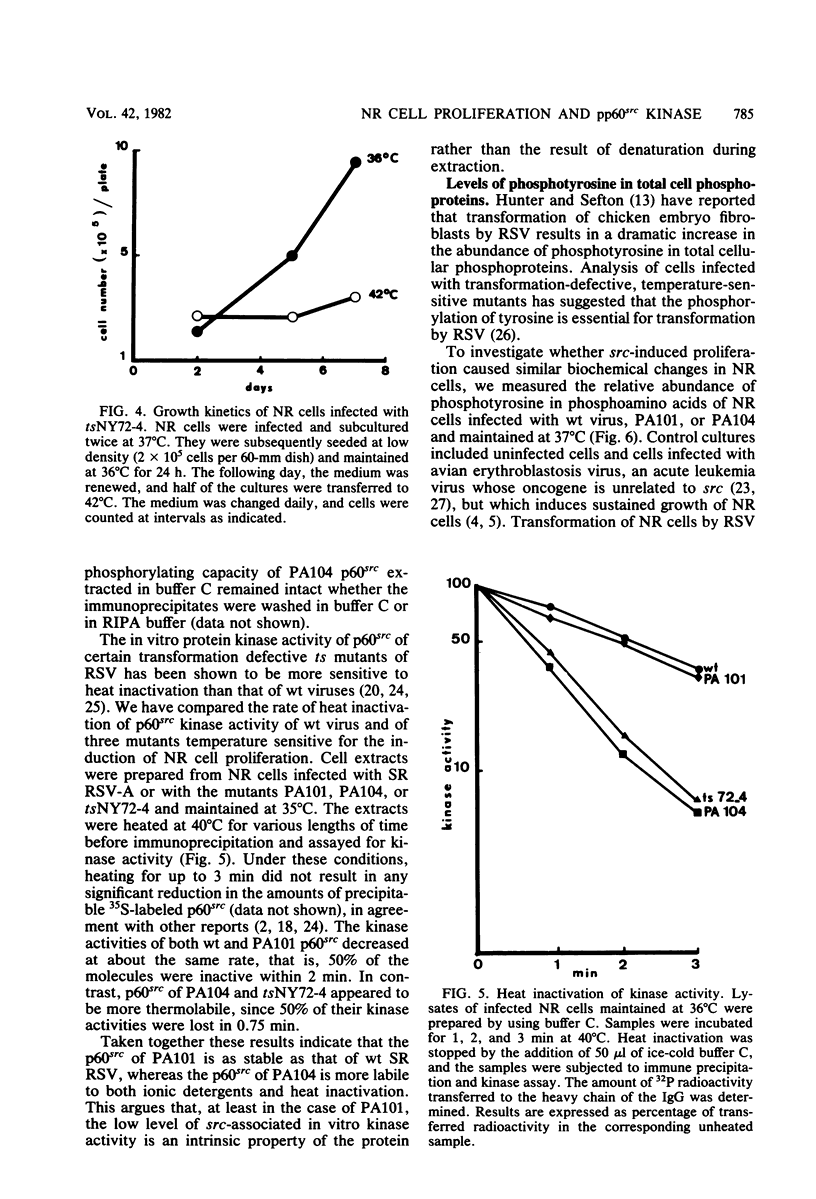
Expression of the src gene of Rous sarcoma virus (RSV) in chicken embryo neuroretinal (NR) cells results in morphological transformation and sustained proliferation of a normally resting cell population. We have previously reported the isolation of mutants of RSV which retain full growth-promoting activity while displaying reduced transforming properties. Two such mutants, PA101 and PA104, were used to investigate whether the p60src-associated kinase activity is required for the mitogenic function of src. A comparison of the patterns of phosphorylation of wild-type and mutant p60src revealed that the phosphorylation of tyrosine residues of p60src of PA104 was markedly reduced, whereas the relative amount of phosphotyrosine in p60src of PA101 was comparable to that of the wild-type protein. In vitro kinase activity of p60src immunoprecipitated from NR cells infected with PA101 or PA104 as measured by phosphorylation of the heavy chains of specific immunoglobulin G molecules was 1/10 that of the wild-type molecule. Moreover, when NR cells infected with mutants temperature sensitive for mitogenic capacity were maintained at a temperature either permissive or restrictive for cell growth, quantitation of kinase activity indicated that proliferation of NR cells could not be linked to the absolute level of in vitro kinase activity of p60src. Transformation of NR cells by wild-type RSV resulted in a 10-fold increase in total cellular phosphotyrosine and in the phosphorylation of tyrosine residues of a 34K protein, a possible in vivo substrate for p60src. In contrast, phosphorylation of tyrosine residues of cellular targets was markedly reduced in NR cells infected with PA101 or PA104. These results indicate that the mitogenic capacity of RSV in NR cells does not require elevated levels of p60src kinase activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- Calothy G., Pessac B. Growth stimulation of chicl embryo neuroretinal cells infected with Rous sarcoma virus: relationship to viral replication and morphological transformation. Virology. 1976 May;71(1):336–345. doi: 10.1016/0042-6822(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Mignatti P., Combes P., Pessac B. Expression of viral oncogenes in differentiating chick embryo neuroretinal cells infected with avian tumor viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):983–990. doi: 10.1101/sqb.1980.044.01.106. [DOI] [PubMed] [Google Scholar]
- Calothy G., Poirier F., Dambrine G., Pessac B. A transformation defective mutant of Rous sarcoma virus inducing chick embryo neuroretinal cell proliferation. Virology. 1978 Aug;89(1):75–84. doi: 10.1016/0042-6822(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Levine A. J. The group C adenovirus tumor antigens: identification in infected and transformed cells and a peptide map analysis. Cell. 1977 Aug;11(4):871–879. doi: 10.1016/0092-8674(77)90298-7. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Owada M., Moelling K. Temperature-sensitive kinase activity associated with various mutants of avian sarcoma viruses which are temperature sensitive for transformation. Virology. 1980 Feb;101(1):157–168. doi: 10.1016/0042-6822(80)90492-4. [DOI] [PubMed] [Google Scholar]
- Pessac B., Calothy G. Transformation of chick embryo neuroretinal cells by Rous sarcoma virus in vitro: induction of cell proliferation. Science. 1974 Aug;185(4152):709–710. doi: 10.1126/science.185.4152.709. [DOI] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Ziemiecki A., Friis R. R., Bauer H. The expression of pp60src and its associated protein kinase activity in cells infected with different transformation-defective temperature-sensitive mutants of Rous sarcoma virus. Virology. 1980 Apr 30;102(2):453–457. doi: 10.1016/0042-6822(80)90113-0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Graf T. Avian myelocytomatosis and erythroblastosis viruses lack the transforming gene src of avian sarcoma viruses. Cell. 1978 Apr;13(4):745–750. doi: 10.1016/0092-8674(78)90224-6. [DOI] [PubMed] [Google Scholar]