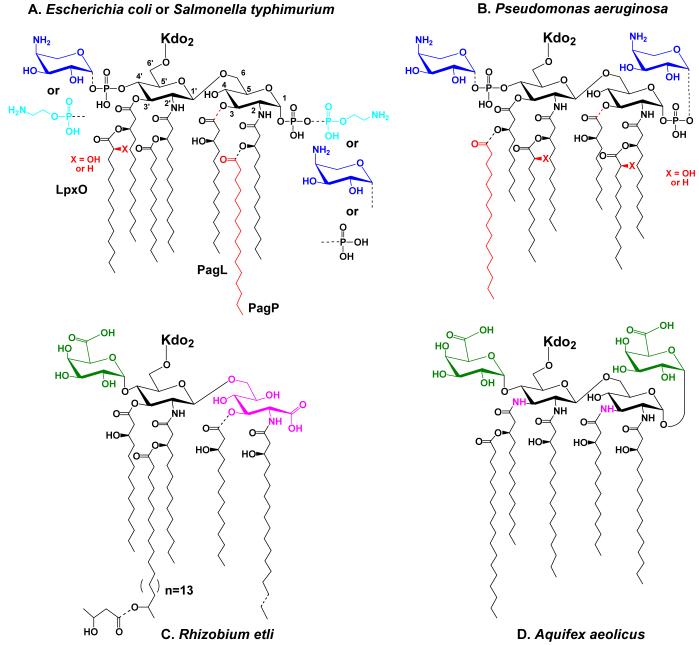
Figure 4. Regulated modifications of lipid A in E. coli and S. typhimurium and unusual lipid A structures in other bacteria.
Partial covalent modifications are indicated with the dashed bonds. Panel A. Modifications of E. coli and S. typhimurium lipid A under the control of PmrA are blue, whereas modifications primarily under the control of PhoP are red. For a recent discussion of the structures of the modified lipid A species that can be isolated from various mutants or under different growth conditions see Zhou et al. (112). When present, the L-Ara4N (dark blue) moiety is located mainly at the 4′ position, whereas the phosphoethanolamine (light blue) is mostly at position 1. Certain lipid A species exist in which the same substituents are attached at both sites, or in which their locations are reversed (112, 370). In cells grown with 1-10 mM Mg++ above pH 7.4, the modifications are suppressed, and a pyrophosphate group (the origin of which is unknown) is present at position 1 in about one third of the lipid A molecules (112). Panel B. Lipid A modifications in Pseudomonas under the control of PmrA are shown in blue, whereas modifications primarily under the control of PhoP are red (109). The portion of the lipid A molecule generated by the constitutive pathway is shown in black. Panel C. R. etli lipid A, which lacks phosphate groups, includes a major species in which aminogluconate (magenta) replaces the proximal glucosamine. Aminogluconate is formed in the outer membrane from a lipid A species containing glucosamine (149, 150, 156). The 4′ galacturonic acid moiety is green. Panel D. Like R. etli, Aquifex aeolicus lipid A lacks phosphate moieties (126), but contains two galacturonic acid residues (green). The hydroxyacyl chains at positions 3 and 3′ differ from E. coli in that they are amide-linked (magenta NH atoms).