Table 1.
Alkyl-Alkyl Suzuki Cross-Coupling of an Unactivated Secondary Alkyl Bromide: Effect of Reaction Parameters 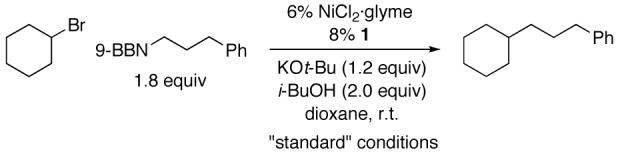
| entry | variation from the “standard” conditions | yield (%) a |
|---|---|---|
| 1 | none | 83 |
| 2 | bathophenanthroline, instead of 1 | 25 |
| 3 | trans-2-aminocyclohexanol, instead of 1 | <5 |
| 4 | prolinol, instead of 1 | <5 |
| 5 | s-Bu-Pybox, b instead of 1 | 7 |
| 6 | trans-1,2-cyclohexanediamine, instead of 1 | 53 |
| 7 | trans-N,N,N’,N’-tetramethyl-1,2-cyclohexanediamine, instead of 1 | <5 |
| 8 | cis isomer of 1, instead of 1 | 46 |
| 9 | N,N’-dimethylethylenediamine, instead of 1 | 7 |
| 10 | no 1 | <5 |
| 11 | no NiCl2·glyme | <5 |
| 12 | NiBr2·diglyme, instead of NiCl2·glyme | 81 |
| 13 | Ni(cod)2, instead of NiCl2·glyme | 75 |
| 14 | NiCl2, instead of NiCl2·glyme | <5 |
| 15 | THF, instead of dioxane | 80 |
| 16 | toluene, instead of dioxane | 47 |
| 17 | Et2O, instead of dioxane | <5 |
| 18 | 3% NiCl2·glyme and 4% 1, instead of 6% NiCl2·glyme and 8% 1 | 56 |
| 19 | no KOt-Bu | <5 |
| 20 | no i-BuOH | <5 |
Determined by GC analysis versus a calibrated internal standard (average of two experiments).
s-Bu-Pybox = (S,S)-2,6-bis(4-(2- butyl)-2-oxazolin-2-yl)pyridine.
