Abstract
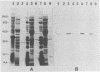
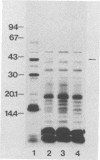
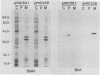
Protein D is an immunoglobulin D-binding membrane protein exposed on the surface of the gram-negative bacterium Haemophilus influenzae. Results reported here indicate that protein D is a lipoprotein. The protein is apparently synthesized as a precursor with an 18-residue-long signal sequence modified by the covalent attachment of both ester-linked and amide-linked palmitate to the cysteine residue, which becomes the amino terminus after cleavage of the signal sequence. Globomycin inhibited maturation of protein D in H. influenzae, implying that protein D is exported through the lipoprotein export pathway. A mutant expressing a protein D lacking the cysteine residue was constructed by oligonucleotide site-directed mutagenesis. The mutated protein D molecule was not acylated and partitioned in the aqueous phase after Triton X-114 extraction of intact bacteria, unlike native and recombinant protein D, which partitioned in the detergent phase. The nonacylated protein D molecule was localized to the periplasmic space of Escherichia coli. The hydrophilic protein D molecule will be used in investigations concerning its ability to function as a vaccine component.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akkoyunlu M., Ruan M., Forsgren A. Distribution of protein D, an immunoglobulin D-binding protein, in Haemophilus strains. Infect Immun. 1991 Apr;59(4):1231–1238. doi: 10.1128/iai.59.4.1231-1238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Biesert L., Scheuer W., Bessler W. G. Interaction of mitogenic bacterial lipoprotein and a synthetic analogue with mouse lymphocytes. Isolation and characterization of binding proteins. Eur J Biochem. 1987 Feb 2;162(3):651–657. doi: 10.1111/j.1432-1033.1987.tb10687.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Anilionis A., Fulginiti J., Metcalf B. J., Quataert S., Quinn-Dey T., Zlotnick G. W., Green B. A. Antigenic conservation of the 15,000-dalton outer membrane lipoprotein PCP of Haemophilus influenzae and biologic activity of anti-PCP antisera. Infect Immun. 1990 Oct;58(10):3388–3393. doi: 10.1128/iai.58.10.3388-3393.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F. D., Smith J., Villacreses N., Metcalf E. S. Polyclonal activation of the murine immune system by an antibody to IgD. VII. Demonstration of the role of nonantigen-specific T help in in vivo B cell activation. J Immunol. 1984 Aug;133(2):550–555. [PubMed] [Google Scholar]
- Forsgren A., Grubb A. O. Many bacterial species bind human IgD. J Immunol. 1979 Apr;122(4):1468–1472. [PubMed] [Google Scholar]
- Forsgren A., Penta A., Schlossman S. F., Tedder T. F. Branhamella catarrhalis activates human B lymphocytes following interactions with surface IgD and class I major histocompatibility complex antigens. Cell Immunol. 1988 Mar;112(1):78–88. doi: 10.1016/0008-8749(88)90277-8. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Osterholm M. T. Safety and efficacy of Haemophilus influenzae type b polysaccharide vaccine. Pediatrics. 1987 Oct;80(4):590–592. [PubMed] [Google Scholar]
- Green B. A., Metcalf B. J., Quinn-Dey T., Kirkley D. H., Quataert S. A., Deich R. A. A recombinant non-fatty acylated form of the Hi-PAL (P6) protein of Haemophilus influenzae elicits biologically active antibody against both nontypeable and type b H. influenzae. Infect Immun. 1990 Oct;58(10):3272–3278. doi: 10.1128/iai.58.10.3272-3278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. A., Quinn-Dey T., Zlotnick G. W. Biologic activities of antibody to a peptidoglycan-associated lipoprotein of Haemophilus influenzae against multiple clinical isolates of H. influenzae type b. Infect Immun. 1987 Dec;55(12):2878–2883. doi: 10.1128/iai.55.12.2878-2883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Janson H., Hedén L. O., Grubb A., Ruan M. R., Forsgren A. Protein D, an immunoglobulin D-binding protein of Haemophilus influenzae: cloning, nucleotide sequence, and expression in Escherichia coli. Infect Immun. 1991 Jan;59(1):119–125. doi: 10.1128/iai.59.1.119-125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lees A., Morris S. C., Thyphronitis G., Holmes J. M., Inman J. K., Finkelman F. D. Rapid stimulation of large specific antibody responses with conjugates of antigen and anti-IgD antibody. J Immunol. 1990 Dec 1;145(11):3594–3600. [PubMed] [Google Scholar]
- Loeb M. R. Protection of infant rats from Haemophilus influenzae type b infection by antiserum to purified outer membrane protein a. Infect Immun. 1987 Nov;55(11):2612–2618. doi: 10.1128/iai.55.11.2612-2618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987 Jan-Feb;9(1):1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S. R., Pichichero M. E. Hemophilus influenzae type b disease. An epidemiologic study with special reference to day-care centers. JAMA. 1984 Nov 9;252(18):2581–2584. doi: 10.1001/jama.252.18.2581. [DOI] [PubMed] [Google Scholar]
- Ruan M. R., Akkoyunlu M., Grubb A., Forsgren A. Protein D of Haemophilus influenzae. A novel bacterial surface protein with affinity for human IgD. J Immunol. 1990 Nov 15;145(10):3379–3384. [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E. D., Capobianco L. A., Berg A. T., Zitt M. Q. The immunogenicity of Hemophilus influenzae type B polysaccharide-Neisseria meningitidis group B outer membrane protein complex vaccine in infants and young children. J Infect Dis. 1989 Dec;160(6):1064–1067. doi: 10.1093/infdis/160.6.1064. [DOI] [PubMed] [Google Scholar]
- Swancutt M. A., Riley B. S., Radolf J. D., Norgard M. V. Molecular characterization of the pathogen-specific, 34-kilodalton membrane immunogen of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3314–3323. doi: 10.1128/iai.57.11.3314-3323.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Weiner M. P., Hutton C. J., Batt C. A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988 May 15;65(1):129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]
- Weinberg G. A., Towler D. A., Munson R. S., Jr Lipoproteins of Haemophilus influenzae type b. J Bacteriol. 1988 Sep;170(9):4161–4164. doi: 10.1128/jb.170.9.4161-4164.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- YOSHIDA A. PURIFICATION AND CHEMICAL CHARACTERIZATION OF MALATE DEHYDROGENASE OF BACILLUS SUBTILIS. J Biol Chem. 1965 Mar;240:1113–1117. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]