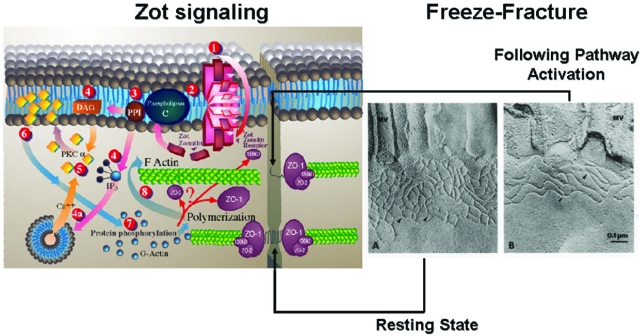
Figure 4.
Proposed zonulin intracellular signaling leading to the opening of intestinal TJ. Zonulin interacts with a specific surface receptor (1) whose distribution within the intestine varies. The protein then activates phospholipase C (2) that hydrolyzes phosphatidyl inositol (3) to release inositol 1,4,5-Tris phosphate (PPI-3) and diacylglycerol (DAG) (4). PKC-α is then activated (5), either directly (via DAG) (4) or through the release of intracellular Ca2+ (via PPI-3) (4a). Membrane-associated, activated PKC-α (6) catalyzes the phosphorylation of target protein(s), with subsequent polymerization of soluble G-actin in F-actin (7). This polymerization causes the rearrangement of the filaments of actin and the subsequent displacement of proteins (including ZO-1) from the junctional complex (8). As a result, intestinal TJs become looser (see freeze fracture electron microscopy). Once zonulin signaling is over, the TJs resume their baseline steady state. Portions of this figure have been reused with permission from Proceedings of the National Academy of Sciences U.S.A. (1991, 88:5242–5246) and Molecular Genetics and Metabolism (1998, 64:12–18).