Abstract
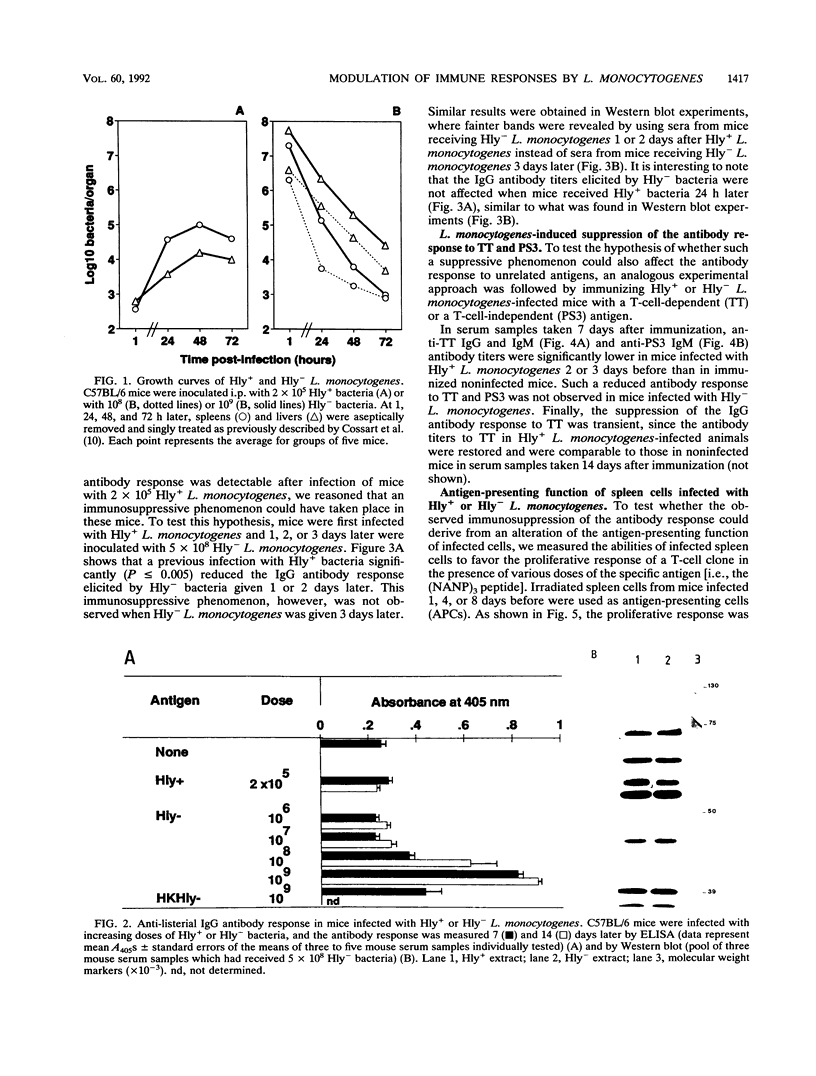
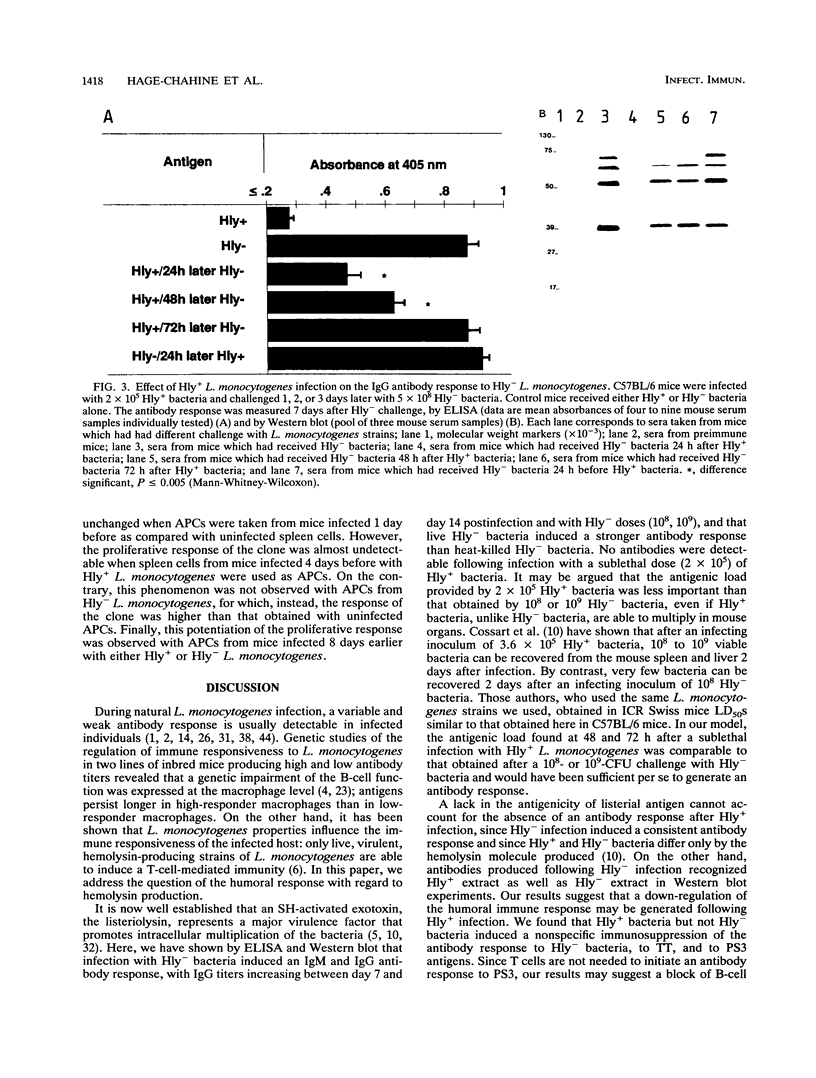
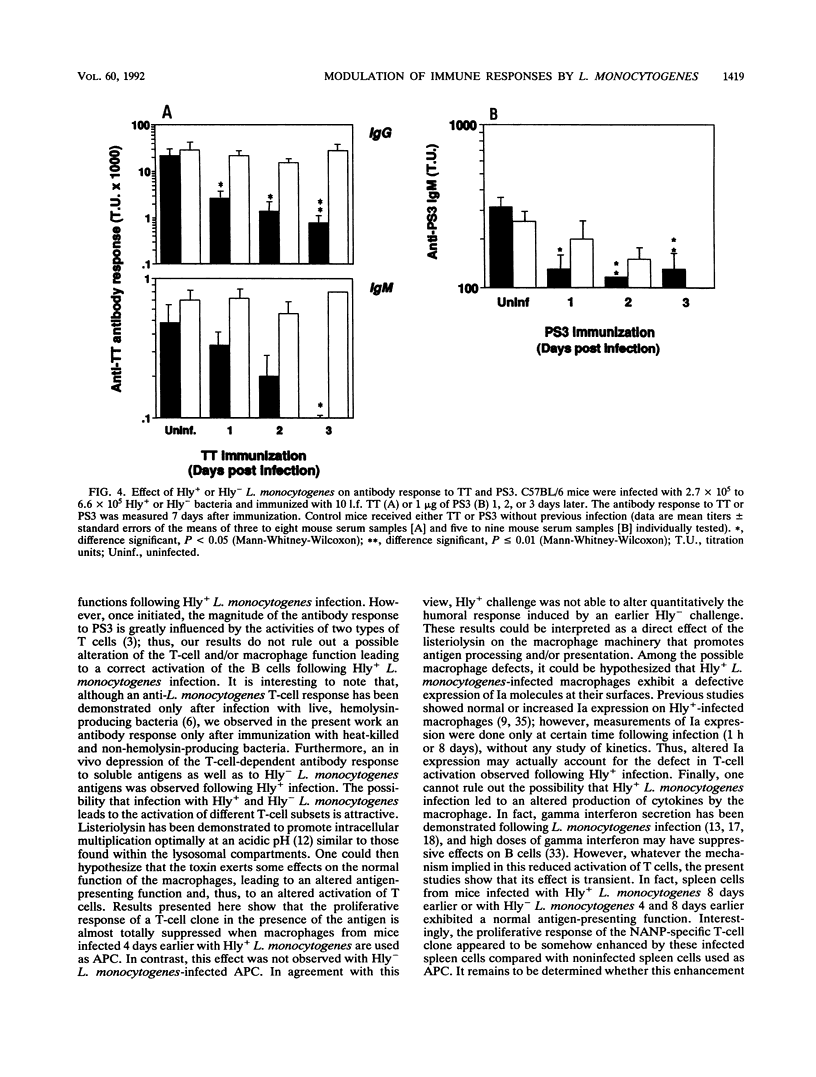
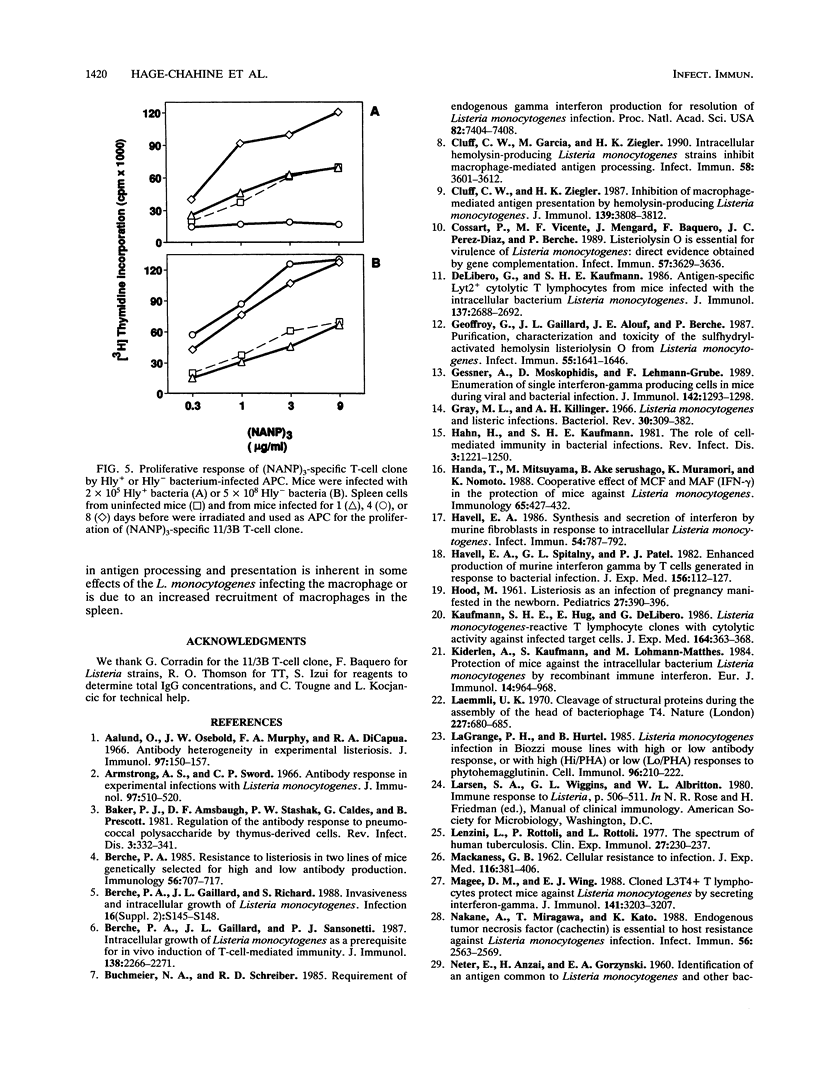
A murine experimental infection with a hemolysin-producing (Hly+) strain of Listeria monocytogenes and a non-hemolysin-producing (Hly-) mutant was used as an in vivo model to evaluate the role of hemolysin production in the immune response. No antilisterial antibodies were detectable following sublethal infection with Hly+ bacteria, but consistent antilisterial immunoglobulin G (IgG) and IgM antibody production was observed following sublethal infection with the Hly- mutant. Hly+ but not Hly- L. monocytogenes induced transient inhibition of antibody response to Hly- bacteria and to unrelated T-cell-dependent (tetanus toxoid) and T-cell-independent (pneumococcal polysaccharide 3) antigens. Transient inhibition of the activation of an antigen-specific T-cell clone was also observed following Hly+ infection of antigen-presenting cells but not following Hly- infection. These results suggest that hemolysin production by L. monocytogenes is an important factor in modulating the immune response to T-cell-dependent and T-cell-independent antigens in infected individuals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalund O., Osebold J. W., Murphy F. A., Di Capua R. A. Antibody heterogeneity in experimental listeriosis. J Immunol. 1966 Jul;97(1):150–157. [PubMed] [Google Scholar]
- Baker P. J., Amsbaugh D. F., Stashak P. W., Caldes G., Prescott B. Regulation of the antibody response to pneumococcal polysaccharide by thymus-derived cells. Rev Infect Dis. 1981 Mar-Apr;3(2):332–341. doi: 10.1093/clinids/3.2.332. [DOI] [PubMed] [Google Scholar]
- Berche P. A. Resistance to listeriosis in two lines of mice genetically selected for high and low antibody production. Immunology. 1985 Dec;56(4):707–716. [PMC free article] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Richard S. Invasiveness and intracellular growth of Listeria monocytogenes. Infection. 1988;16 (Suppl 2):S145–S148. doi: 10.1007/BF01639738. [DOI] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluff C. W., Garcia M., Ziegler H. K. Intracellular hemolysin-producing Listeria monocytogenes strains inhibit macrophage-mediated antigen processing. Infect Immun. 1990 Nov;58(11):3601–3612. doi: 10.1128/iai.58.11.3601-3612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluff C. W., Ziegler H. K. Inhibition of macrophage-mediated antigen presentation by hemolysin-producing Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3808–3812. [PubMed] [Google Scholar]
- Cossart P., Vicente M. F., Mengaud J., Baquero F., Perez-Diaz J. C., Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989 Nov;57(11):3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner A., Moskophidis D., Lehmann-Grube F. Enumeration of single IFN-gamma-producing cells in mice during viral and bacterial infection. J Immunol. 1989 Feb 15;142(4):1293–1298. [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOD M. Listeriosis as an infection of pregnancy manifested in the newborn. Pediatrics. 1961 Mar;27:390–396. [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Handa T., Mitsuyama M., Serushago B. A., Muramori K., Nomoto K. Co-operative effect of MCF and MAF(IFN-gamma) in the protection of mice against Listeria monocytogenes. Immunology. 1988 Nov;65(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L., Patel P. J. Enhanced production of murine interferon gamma by T cells generated in response to bacterial infection. J Exp Med. 1982 Jul 1;156(1):112–127. doi: 10.1084/jem.156.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A. Synthesis and secretion of interferon by murine fibroblasts in response to intracellular Listeria monocytogenes. Infect Immun. 1986 Dec;54(3):787–792. doi: 10.1128/iai.54.3.787-792.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- LaGrange P. H., Hurtrel B. Listeria monocytogenes infection in Biozzi mouse lines with high or low antibody responses, or with high (Hi/PHA) or low (Lo/PHA) responses to phytohemagglutinin. Cell Immunol. 1985 Nov;96(1):210–222. doi: 10.1016/0008-8749(85)90352-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988 Nov 1;141(9):3203–3207. [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman R. E., Lorber B. Listeriosis in adults: a changing pattern. Report of eight cases and review of the literature, 1968-1978. Rev Infect Dis. 1980 Mar-Apr;2(2):207–227. doi: 10.1093/clinids/2.2.207. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., DiCapua R. A. Cellular immunity of mice infected with Listeria monocytogenes in diffusion chambers. J Bacteriol. 1968 Jun;95(6):2158–2164. doi: 10.1128/jb.95.6.2158-2164.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. S., Boom W. H., Abbas A. K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987 Aug 1;139(3):767–773. [PubMed] [Google Scholar]
- Rodriguez G. E., McClatchy J. K., Campbell P. A. Induction of Resistance by Listeria monocytogenes Cell Wall Fraction. Infect Immun. 1974 Nov;10(5):1163–1169. doi: 10.1128/iai.10.5.1163-1169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Interaction between phagocytes and pathogenic microorganisms. Bacteriol Rev. 1956 Jun;20(2):94–132. doi: 10.1128/br.20.2.94-132.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safley S. A., Cluff C. W., Marshall N. E., Ziegler H. K. Role of listeriolysin-O (LLO) in the T lymphocyte response to infection with Listeria monocytogenes. Identification of T cell epitopes of LLO. J Immunol. 1991 May 15;146(10):3604–3616. [PubMed] [Google Scholar]
- Sansonetti P., Lagrange P. H. The immunology of leprosy: speculations on the leprosy spectrum. Rev Infect Dis. 1981 May-Jun;3(3):422–469. doi: 10.1093/clinids/3.3.422. [DOI] [PubMed] [Google Scholar]
- Seeliger H. P. Etat actuel de nos connaissances sur les Listeria et les listérioses. Bull Acad Natl Med. 1987 Oct;171(7):799–806. [PubMed] [Google Scholar]
- Togna A. R., Del Giudice G., Verdini A. S., Bonelli F., Pessi A., Engers H. D., Corradin G. Synthetic Plasmodium falciparum circumsporozoite peptides elicit heterogenous L3T4+ T cell proliferative responses in H-2b mice. J Immunol. 1986 Nov 1;137(9):2956–2960. [PubMed] [Google Scholar]
- Visintine A. M., Oleske J. M., Nahmias A. J. Infection in infants and children. Am J Dis Child. 1977 Apr;131(4):393–397. doi: 10.1001/archpedi.1977.02120170019002. [DOI] [PubMed] [Google Scholar]
- WELSHIMER H. J. Staphylococcal antibody production in response to injections with Listeria monocytogenes. J Bacteriol. 1960 Mar;79:456–457. doi: 10.1128/jb.79.3.456-457.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters W. D., Cox R. A. Serodiagnosis of tuberculosis by radioimmunoassay. Am Rev Respir Dis. 1981 Nov;124(5):582–585. doi: 10.1164/arrd.1981.124.5.582. [DOI] [PubMed] [Google Scholar]
- Yamada A., Himeno K., Nakamura S., Kawamura I., Nomoto K. Transfer of resistance to primary infection of Listeria monocytogenes and early induction of delayed hypersensitivity by sera from L. monocytogenes-infected mice. Infect Immun. 1987 Dec;55(12):3078–3084. doi: 10.1128/iai.55.12.3078-3084.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




