Figure 6.
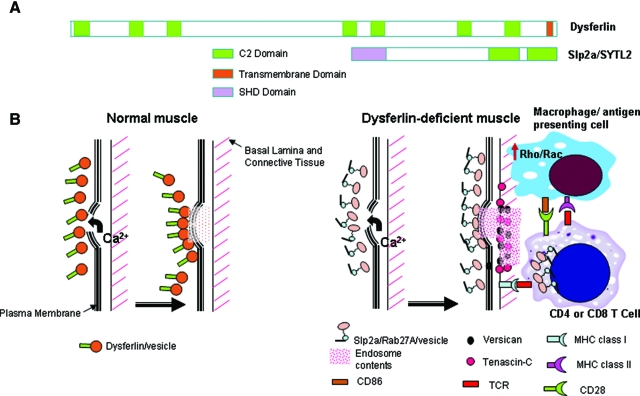
Model for Effective Compensatory Membrane Repair, but Proinflammatory Milieu in Dysferlin Deficient Muscle. A: Shown is the dysferlin protein (230kDa) with seven putative C2 domains and a C-terminal transmembrane domain (TM), compared to the Slp2a/SYTL2 protein (110kDa) containing two C2 domains. Slp2a contains an extra SHD domain, that is absent in dysferlin. The SHD domain is important for binding GTP-bound activated form of Rab proteins on vesicles, where the complex then participates in vesicle trafficking pathways. Protein schematics are drawn to relative scale. B: Shown is a schematic of plasma membrane repair in normal muscle following membrane damage, and a model of consequences of compensatory up-regulation of the Slp2a/Rab27A pathway. In normal myofibers, dysferlin plays a major role in vesicle traffic and membrane repair in response to calcium influx. In dysferlin-deficient myofibers, the Slp2a/Rab27A complex is highly expressed, presumably to compensate for lack of dysferlin-linked membrane repair mechanisms. This may effectively repair myofiber membrane damage and no symptoms for two decades. We hypothesize that four interrelated processes may combine to drive disease onset and progression: 1) release of Slp2a/Rab27A exocytotic vesicle contents (pink diamonds); 2) increased Rho/Rac signaling in dysferlin-deficient macrophages lead to activation and inappropriate phagocytotic activity; 3) possible positive feedback between the T cell Slp2a/Rab27A-mediated lytic granule cell killing, and up-regulated Slp2a/Rab27A; and 4) possible interaction between activated macrophages/dendritic cells with CD4 T cells via signal 1 (MHC class II and CD4) and signal 2 (CD86 and CD28) as well as interaction between CD8 T cells and MHC class I on muscle lead to further immune activation. The observed focal increases in versican and tenascin-C may contribute to, or simply reflect the pro-inflammatory milieu.