Abstract
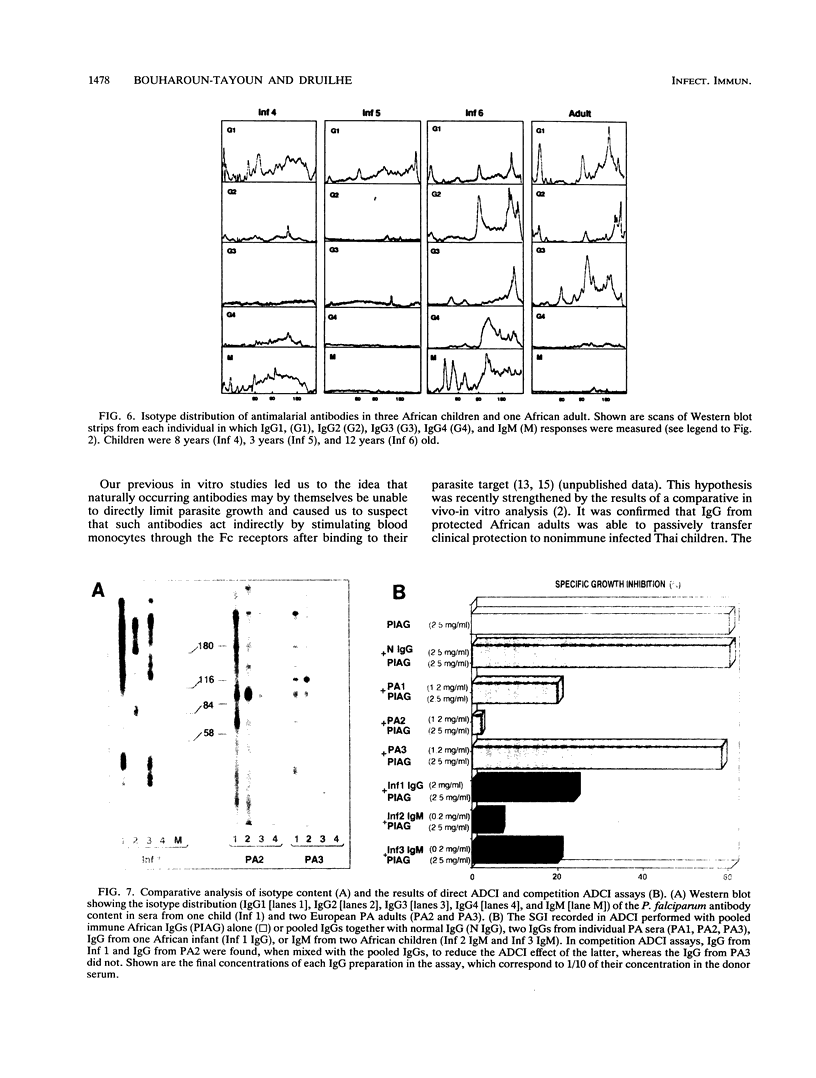
In view of the recent demonstration that antibodies that are protective against Plasmodium falciparum malaria may act in collaboration with blood monocytes, we investigated the isotype content of sera from individuals with defined clinical states of resistance or susceptibility to malaria. Profound differences in the distribution of each immunoglobulin subclass were found. Immunoglobulin G1 (IgG1) and IgG3, two cytophilic isotypes, predominated in protected subjects. In nonprotected subjects, i.e., children and adults that have sustained a primary malarial attack, four different situations were encountered: (i) an imbalance in which IgG2, a noncytophilic class, predominated (mostly seen in primary attacks), (ii) an imbalance also concerning IgG2 but only of a given antigenic specificity, (iii) an imbalance in which mostly IgM antibodies predominated (a frequent event in children), and, less frequently, (iv) an overall low level of antimalarial antibodies. Of 33 nonimmune subjects studied, all but one had one of the above defects. The function of total immunoglobulin presenting such an isotype imbalance was studied in vitro in antibody-dependent cellular inhibition assays. IgG from protected subjects cooperated efficiently with blood monocytes, whereas IgG from nonprotected groups did not. Also, the latter could inhibit the in vitro effect of the former: in competition assays whole IgG from primary-attack cases with increased IgG2 content and IgG or IgM from children from endemic areas competed with IgG from immune adults. This led us to formulate the hypothesis that nonprotected subjects have antibodies to epitopes critical for protection, but that these antibodies are nonfunctional. These results bring some clues to the very long delay required to reach protection against malaria and clearly stress the need to investigate immune responses in both quantitative and qualitative terms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aucouturier P., Mounir S., Preud'homme J. L. Distribution of IgG subclass levels in normal adult sera as determined by a competitive enzyme immunoassay using monoclonal antibodies. Diagn Immunol. 1985;3(4):191–196. [PubMed] [Google Scholar]
- Bouharoun-Tayoun H., Attanath P., Sabchareon A., Chongsuphajaisiddhi T., Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990 Dec 1;172(6):1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Ouma J. H., Butterworth A. E. Immunity to schistosomes: progress toward vaccine. Science. 1987 Nov 20;238(4830):1065–1072. doi: 10.1126/science.3317823. [DOI] [PubMed] [Google Scholar]
- Druilhe P., Khusmith S. Epidemiological correlation between levels of antibodies promoting merozoite phagocytosis of Plasmodium falciparum and malaria-immune status. Infect Immun. 1987 Apr;55(4):888–891. doi: 10.1128/iai.55.4.888-891.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne D. W., Grabowska A. M., Fulford A. J., Butterworth A. E., Sturrock R. F., Koech D., Ouma J. H. Human antibody responses to Schistosoma mansoni: the influence of epitopes shared between different life-cycle stages on the response to the schistosomulum. Eur J Immunol. 1988 Jan;18(1):123–131. doi: 10.1002/eji.1830180119. [DOI] [PubMed] [Google Scholar]
- Falanga P. B., D'Imperio Lima M. R., Coutinho A., Pereira da Silva L. Isotypic pattern of the polyclonal B cell response during primary infection by Plasmodium chabaudi and in immune-protected mice. Eur J Immunol. 1987 May;17(5):599–603. doi: 10.1002/eji.1830170504. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Groux H., Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol. 1990 Jul-Aug;141(6):529–542. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Khalife J., Dunne D. W., Richardson B. A., Mazza G., Thorne K. J., Capron A., Butterworth A. E. Functional role of human IgG subclasses in eosinophil-mediated killing of schistosomula of Schistosoma mansoni. J Immunol. 1989 Jun 15;142(12):4422–4427. [PubMed] [Google Scholar]
- Khusmith S., Druilhe P. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect Immun. 1983 Jul;41(1):219–223. doi: 10.1128/iai.41.1.219-223.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lunel F., Druilhe P. Effector cells involved in nonspecific and antibody-dependent mechanisms directed against Plasmodium falciparum blood stages in vitro. Infect Immun. 1989 Jul;57(7):2043–2049. doi: 10.1128/iai.57.7.2043-2049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R. J., Carson D. C., Greenwood B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989 May-Jun;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Morell A., Skvaril F., Hitzig W. H., Barandun S. IgG subclasses: development of the serum concentrations in "normal" infants and children. J Pediatr. 1972 Jun;80(6):960–964. doi: 10.1016/s0022-3476(72)80007-6. [DOI] [PubMed] [Google Scholar]
- Quinti I., Papetti C., von Hunolstein C., Orefici G., Aiuti F. IgG subclasses to group B streptococci in normals, colonized woman and IgG2 subclass-deficient patients. Monogr Allergy. 1988;23:148–155. [PubMed] [Google Scholar]
- Reese R. T., Langreth S. G., Trager W. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull World Health Organ. 1979;57 (Suppl 1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Riley E. M., Jobe O., Whittle H. C. CD8+ T cells inhibit Plasmodium falciparum-induced lymphoproliferation and gamma interferon production in cell preparations from some malaria-immune individuals. Infect Immun. 1989 Apr;57(4):1281–1284. doi: 10.1128/iai.57.4.1281-1284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson I. A., Hodgen A. N., Arthur I. H. The separation of immunoglobulin M from human serum by fast protein liquid chromatography. J Immunol Methods. 1984 Apr 13;69(1):9–15. doi: 10.1016/0022-1759(84)90271-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Pacheco E., Evans C. B., Asofsky R. Inbred mice infected with Plasmodium yoelii differ in their antimalarial immunoglobulin isotype response. Parasite Immunol. 1988 Jan;10(1):33–46. doi: 10.1111/j.1365-3024.1988.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- WILSON D. B., GARNHAM P. C. C., SWELLENGREBEL N. H. A review of hyperendemic malaria. Trop Dis Bull. 1950 Aug;47(8):677–698. [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983 Oct;54(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Perlmann H., Berzins K., Björkman A., Larsson A., Ljungström I., Patarroy M. E., Perlmann P. Characterization of the humoral immune response in Plasmodium falciparum malaria. III. Factors influencing the coexpression of antibody isotypes (IgM and IgG-1 to 4). Clin Exp Immunol. 1986 Feb;63(2):343–353. [PMC free article] [PubMed] [Google Scholar]
- White W. I., Evans C. B., Taylor D. W. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991 Oct;59(10):3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]