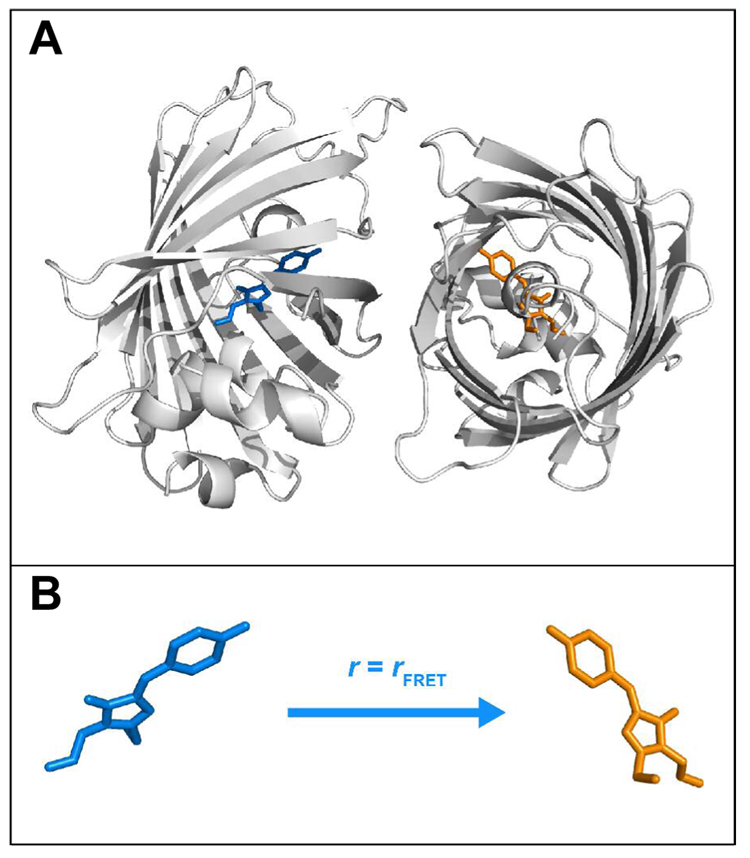
Figure 5.
(A) Structural model of YFP dimer and (B) the corresponding anisotropy of the unidirectional hetero-FRET. Ribbon and stick structures are shown for the protein and the chromophore, respectively. The neutral and anionic chromophores are colored in blue and orange, respectively. The images were created using the coordinates from a crystallographic YFP dimer (PDB code: 1YFP) as follows: one monomer was obtained directly from chain A in the PDB file; for the other monomer, the PyMOL (DeLano Scientific LLC) command, symexp, was executed to generate the symmetry-related object sym02000000, which corresponds to the symmetry operator (1/2 + X, 1/2 − Y, 1 − Z), from chain B in the PDB file; thus, the dimer interface between the two monomers, including residues Ala206, Leu221, and Phe223, which was observed for wild-type GFP (5), YFP 10C (3), and Venus (40) can be visualized (not shown for clarity), while the coordinates of chain B in the PDB file does not produce this interface with chain A. The structure of the YFP dimer in the crystal may not necessarily reflect that in solution, but is a starting point for the quantitative analysis of the hetero-FRET (see text for discussion).