Abstract
The purpose of this study was to examine the influence of reduced tongue activity by artificial rearing on the morphology of motoneurons innervating the extrinsic tongue retrusors. Artificially reared rat pups were fed via gastric cannula from postnatal day 3 to postnatal day 14. Artificially reared animals and dam-reared controls had cholera toxin (subunit B) conjugate of horseradish peroxidase injected into the styloglossus to label motoneurons innervating hyoglossus and styloglossus on postnatal day 13 and postnatal day 59, artificially reared animals and dam-reared controls. Following perfusion on postnatal days 14 and 60, serial transverse sections treated with tetramethyl benzidine and counter-stained neutral red were used to analyze motoneuron morphology. The shorter diameter of hyoglossus motoneurons increased with age for the dam-reared but not the artificially reared group. There was a tendency for a similar pattern for styloglossus motoneurons across the two rearing groups. The changes in form factor reflected the changes in shorter diameter for both motoneuron pools. Therefore, reducing suckling activity during normal postnatal development leads to diminished motoneuron somal growth in rats. This may also be the case in premature infants necessarily fed artificially.
Keywords: Postnatal Development, Suckling, Brainstem, Hypoglossal Nucleus
Introduction
Infants with a variety of health problems are sometimes fed using routes other than oral. This results in reduced tongue use that may contribute to delayed oro-motor development. For example, infants requiring neonatal intensive care display an increased frequency of speech motor disorders (Jennische and Sedin, 1998, 1999) and perinatal infants subjected to prolonged parenteral feeds exhibit impaired suckling abilities at term (Hawdon et al., 2000). The latter problem is of particular significance as it can lead to longer hospital stays (Schanler et al., 1999). In both instances, deficits may be due, at least in part, to the disruption of the normal development of the hypoglossal motor system.
The intrinsic muscles, those muscles contained within the tongue (vertical, transverse, superior and inferior longitudinal), and most of the extrinsic muscles (genioglossus, styloglossus, and hyoglossus) of the tongue are innervated by the hypoglossal nerve. The extrinsic muscles originate from a boney structure such as the mandible (genioglossus), styloid process (styloglossus), or hyoid bone (hyoglossus) and insert onto the body of the tongue. The hypoglossal nucleus is subdivided into two main compartments, the ventral and dorsal compartments. Motoneurons projecting out of the ventral (protrusor) compartment innervate the genioglossus, verticalis, and transversus muscles (Aldes, 1995). The dorsal (retrusor) compartment contains motoneurons that innervate the styloglossus, hyoglossus, and superior and inferior longitudinal muscles (McClung and Goldberg, 1999). In the rat, an accessory portion of the hypoglossal nucleus contains motoneurons innervating the geniohyoid muscle (McClung and Goldberg, 1999).
We recently reported the normal postnatal development of motoneurons innervating the hyoglossus and styloglossus muscles (Smith et al., 2005). There is a bimodal distribution of these motoneurons within the dorsal (retrusor) compartment of the hypoglossal nucleus. Furthermore, hypoglossal neuronal migration in the developing rat nervous system appears to be complete by the first week of life.
There is some evidence to suggest that activity may modulate the normal postnatal development of the hypoglossal motor system. The use of hindlimb suspension in rats from postnatal days 21 to 42 results in smaller soma size and reduced succinate dehydrogenase activity in motoneurons innervating the soleus (Nakano and Katsuta, 2000). The number of axons ramifying in the endplate is also reduced in the soleus when using the hindlimb suspension model from postnatal days 8-17 (Huckstorf et al., 2000). In addition, the use of artificial rearing (‘pup in a cup’ model) from postnatal days 4-14 has demonstrated altered contractile properties of the tongue retrusor musculature (Kinirons et al., 2003). The dendritic pattern, including the number of primary dendrites is altered within 5 days of spinal cord transection in young rats (Gazula et al., 2004). However, the dendritic pattern is no different from a control group when the rats with spinal cord transection exercise performed routine passive hindlimb exercise. This suggests that activity plays a crucial role in the maintenance of the dendritic tree.
Therefore, the purpose of this study was to evaluate the effects of reduced tongue activity on the morphology of motoneurons innervating the styloglossus and hyoglossus in neonatal and adult rat pups. We previously reported physiological changes associated with artificially rearing animals from postnatal days 4 to 14 (Kinirons et al., 2003). Postnatal day 14 was chosen in the current study to show morphological changes that accompany these physiological changes. Postnatal day 60 was chosen because we had already published normal postnatal development of motoneuron morphology in 8-week-old animals (Smith et al., 2005). In this manuscript, we will refer to hypoglossal motoneurons innervating hyoglossus and styloglossus as hyoglossus motoneurons and styloglossus motoneurons, respectively.
Materials and Methods
Virginia Commonwealth University's Institutional Animal Care and Use Committee approved all procedures and protocols for animal care. All animals were housed on a 12-hr light/dark cycle. Following recovery from surgery, the animals were put back with their mothers, put back in a cage, or continued the artificial rearing process until perfusion. Those animals that were capable of eating rat chow had unlimited access to standard rat chow and water.
Experiments were carried out on twenty Sprague-Dawley rats. The timing of the experiment was age-dependent. Ten animals underwent surgery on postnatal day 13 (P13) and the other ten animals underwent surgery on postnatal day 59 (P59), counting their day of birth as postnatal day 1. Half of each age group was artificially reared from postnatal day 3 (P3) to postnatal day 14 (P14). At the conclusion of the artificial rearing process, animals that did not undergo surgery had their cannulae cut close to the abdomen and were put back in the cage with an appropriate surrogate mother until weaning. After weaning, the animals were put in a separate cage from the mother and allowed unlimited access to standard rat chow and water. Perfusion occurred 20-24-h post-surgery for each animal.
Artificial Rearing
On P3, rat pups intended for the artificial rearing process were taken from their mother, anesthetized using isoflurane, and had a gastric cannula inserted into their stomachs using methods previously described (Hall, 1975). After the animals recovered from the cannula implantation procedure, they were raised from P3 to P14 using the ‘pup in a cup’ model. Briefly, the pups were placed in a Styrofoam cup containing bedding. A plastic lid with several holes covered the cup and secured in place with a rubber band to prevent the animal from crawling out. Each cup had a rubber weight mounted to the bottom. The cannula was passed through a central hole in the lid and connected to a feeding line. Each cup floated in a temperature controlled water bath (38-40° C). Every day, the animals were removed from the cups, weighed, cannulae flushed with sterile water, stimulated to urinate and defecate, and placed back in the cup with clean bedding.
Milk and Feeding Schedule
The substitute milk formula used in this experiment was a modification (West et al., 1984) of the Messer diet (Messer et al., 1969). The pups were fed via milk-containing syringes using an automated syringe pump system (Harvard Apparatus, Holliston, MA). The artificially reared animals were fed continuously for 10-min out of every hour in a 24-h period. Feeding volume for each day was determined by the group's mean body weight for that day (Lovic and Fleming, 2004). On P3, the artificially reared animals received a volume equivalent to 33% of their mean body weight and increased ∼1% every day. Every 24-h, the syringes were replaced with fresh milk and the feeding lines flushed with distilled water.
Neural Tracer Injection
A surgical plane of anesthesia was created using either ketamine (60mg/kg, i.p.) and xylazine (7 mg/kg, i.p.) or isoflurane. Depth of anesthesia was assessed by absence of the flexor withdrawal reflex. Using a ventral surgical approach, a midline incision was made along the neck, the styloglossus muscle was exposed, and 0.5 to 5.0 μl of 0.1% cholera toxin (subunit B) conjugate of horseradish peroxidase (CTHRP) was injected into the medial portion of the innervation zone. This injection technique has been shown to isolate labeling of motoneurons within the hypoglossal nucleus that innervate styloglossus and hyoglossus only (Smith et al., 2005).
Perfusion
For perfusion, each animal was deeply anesthetized as previously indicated and killed by transcardial perfusion with a mixed aldehyde fixative. Serial (50 μm) transverse sections of the hypoglossal nucleus were made and CTHRP labeled motoneurons were histochemically localized with tetramethyl benzidine. Labeled sections were mounted, counterstained in neutral red, coverslipped with Permount, and analyzed under brightfield illumination. To control for measurement bias, the age and rearing group for each slide was blinded from the individual analyzing the data until data analysis was complete. A computer image analysis program (Neurolucida, MicroBrightField, Williston, VT) was used to measure long and short diameter, form factor, and to count the number of primary dendrites. Form factor was derived from the relationship between the perimeter and area of the soma. This measure was obtained by tracing the perimeter of the soma and base of the primary dendrites. A form factor value of 1.0 would denote a perfect circle. Motoneurons with at least 4 primary dendrites visible were chosen for analysis. This criterion was chosen to ensure a minimum level of backfilling by CTHRP within the cell to be able to visualize the perimeter of the soma. Care was taken in identifying the same motoneuron in adjacent sections based on its shape and location and not measured more than once.
Statistical Analysis
A 2 × 2 analysis of variance (ANOVA) was used to assess differences across age and rearing groups for each motoneuron pool (hyoglossus and styloglossus motoneurons). Nineteen hyoglossus and 25 styloglossus motoneurons from each age and rearing group were randomly selected for analysis. Level of significance was set at 0.05. Scheffé's multiple comparisons was used to assess differences when the factorial ANOVA resulted in a significant interaction.
Results
There was a tendency for a significant difference for the body weights at P14 (F = 4.644; P = 0.063). The artificially reared pups weighed 19.71 g (standard deviation = 5.61) at P14 while the dam-reared pups weighed 25.47 g (standard deviation = 2.08). By P60, there was no difference between artificially reared animals and dam-reared animals in terms of body weight. The artificially reared animals weighed 226.80 g (standard deviation = 28.73) and the dam-reared animals weighed 239.20 g (standard deviation = 41.12).
Hyoglossus Motoneurons
Labeled hyoglossus motoneurons were found in the same approximate location in dam reared and artificially reared animals. For P14, hyoglossus motoneurons were found ∼400μm caudal to obex to ∼200μm rostral to the obex in dam reared animals and ∼350μm caudal to obex to ∼100μm rostral to the obex in artificially reared animals. The obex is defined as the junction of the fourth ventricle and the central canal in the transverse plane. For animals aged postnatal day 60 (P60), hyoglossus motoneurons were found ∼500μm caudal to obex to ∼150μm rostral to the obex in dam reared animals and ∼600μm caudal to obex to ∼200μm rostral to the obex in the artificially reared animals.
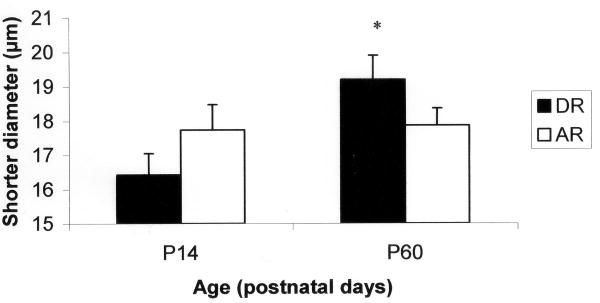
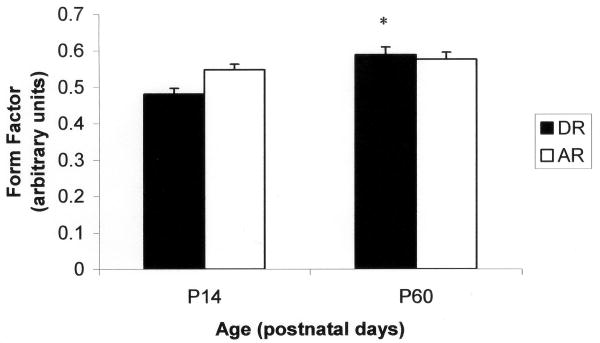
The means and standard errors for all cell measurements for hyoglossus motoneurons are listed in Table 1. The results of the factorial ANOVA demonstrated main effects for age in the number of primary dendrites (F = 6.328; P = 0.014) and mean diameter (F = 5.430; P = 0.023) for hyoglossus motoneurons. There was a tendency for a main effect for age in the longer diameter (F = 3.122; P = 0.081). Significant interaction effects were found for the shorter diameter (F = 3.994; P = 0.049) and form factor (F = 4.533; P = 0.037). Post hoc analysis revealed that the shorter diameter increased with age in the dam reared group (F = 2.933; P = 0.021) but not the artificially reared group (Figure 1). In addition, the shape of the soma became more circular as the animal increased in age for the dam reared group (F = 6.701; P = 0.001) but not the artificially reared group (Figure 2).
Table 1.
Cell Measurements for Hyoglossus Motoneurons.
| Age | Variable | Dam Reared | Artificially Reared |
|---|---|---|---|
| P14 | |||
| Primary Dendrites | 4 (0.13)1 | 4 (0.19)1 | |
| Long Diameter (μm) | 24.60 (1.00) | 24.29 (1.38) | |
| Short Diameter (μm) | 16.43 (0.62)1 | 17.74 (0.74) | |
| Mean Diameter (μm) | 20.52 (0.65)1 | 21.02 (0.99)1 | |
| Form Factor | 0.4816 (0.0160)1 | 0.5474 (0.0154) | |
| P60 | |||
| Primary Dendrites | 5 (0.20) | 5 (0.18) | |
| Long Diameter (μm) | 26.95 (0.93) | 25.96 (1.20) | |
| Short Diameter (μm) | 19.19 (0.71) | 17.87 (0.50) | |
| Mean Diameter (μm) | 23.07 (0.56) | 21.92 (0.70) | |
| Form Factor | 0.5884 (0.0218) | 0.5758 (0.0197) |
Standard error in parentheses.
significantly different from P60 counterpart (P < 0.05)
Figure 1.
Short diameter of hyoglossus motoneurons for dam-reared (DR) and artificially reared (AR) groups at P14 and P60. *significantly different from DR P14 (P = 0.021).
Figure 2.
Hyoglossus motoneuron form factor for artificially reared (AR) and dam-reared (DR) animals at P14 and P60. *significantly different from DR P14 (P = 0.001).
Styloglossus Motoneurons
Labeled styloglossus motoneurons were also found in similar locations between the dam reared and artificially reared animals for each age group. For P14, styloglossus motoneurons were found ∼450-600μm rostral to obex for both rearing groups. For P60, styloglossus motoneurons were found ∼600-900μm rostral to obex for dam reared animals and ∼600-950μm rostral to obex for artificially reared animals.
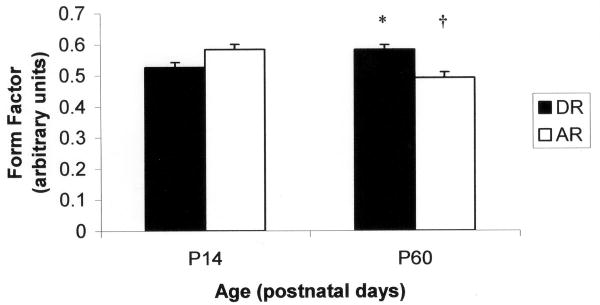
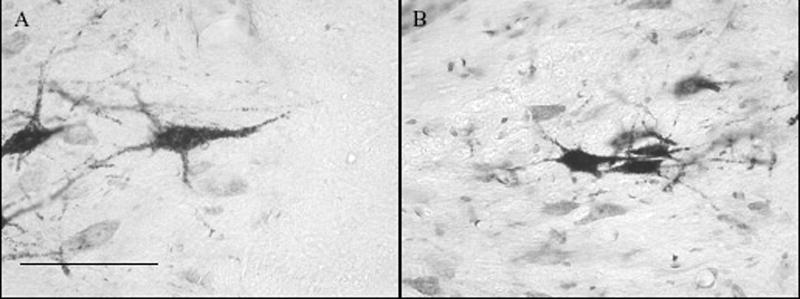
The means and standard errors for all styloglossus motoneuron cell measurements are listed in Table 2. There was no difference in the number of primary dendrites across age or rearing groups. The results of the factorial ANOVA demonstrated main effects for age for the longer diameter (F = 8.312; P = 0.005) and mean diameter (F = 9.465; P = 0.003). There was a tendency for an interaction effect for the shorter diameter (F = 3.750; P = 0.056). In addition, the shape of the soma became less circular as the artificially reared group aged (F = 7.663; P = 0.001) but not the dam reared group (Figure 3). Furthermore, the shape of the soma at P60 for the artificially reared animals was less circular than their P60 dam-reared counterparts (F = 7.663; P = 0.001) (Figure 4).
Table 2.
Cell Measurements for Styloglossus Motoneurons.
| Age | Variable | Dam Reared | Artificially Reared |
|---|---|---|---|
| P14 | |||
| Primary Dendrites | 5 (0.13) | 5 (0.17) | |
| Long Diameter (μm) | 27.96 (0.84)1 | 26.71 (1.27)1 | |
| Short Diameter (μm) | 17.85 (0.77) | 18.72 (0.51) | |
| Mean Diameter (μm) | 22.90 (0.56)1 | 22.71 (0.76)1 | |
| Form Factor | 0.5256 (0.0158) | 0.5832 (0.0166)1 | |
| P60 | |||
| Primary Dendrites | 5 (0.16) | 4 (0.10) | |
| Long Diameter (μm) | 30.41 (1.07) | 30.50 (1.12) | |
| Short Diameter (μm) | 20.23 (0.91) | 18.32 (0.62) | |
| Mean Diameter (μm) | 25.32 (0.73) | 24.41 (0.60) | |
| Form Factor | 0.5820 (0.0151) | 0.4908 (0.0178)2 |
Standard error in parentheses.
significantly different from P60 counterpart (P < 0.05)
significantly different from Dam reared P60 (P = 0.001)
Figure 3.
Styloglossus motoneuron form factor for artificially reared (AR) and dam-reared (DR) animals at P14 and P60. †significantly different from AR P14 (P = 0.001). *significantly different from AR P60 (P = 0.001).
Figure 4.
Differences in shape of P60 styloglossus motoneurons between dam-reared (A) and artificially reared rats (B). Bar is 100 μm.
Discussion
The purpose of this investigation was to determine the morphological changes in hyoglossus and styloglossus motoneurons associated with the artificial rearing process. We report two major findings in this study. 1) The artificial rearing process retards hyoglossus motoneuron growth in one direction, and 2) The artificial rearing process disrupts normal postnatal development of the shape of hyoglossus and styloglossus motoneurons.
Body weights
The body weights of the artificially reared pups were ∼77% of the dam-reared body weights at P14. By P60, the artificially reared animals had recovered to ∼95% of the dam-reared body weights. This reduced body weight in the artificially reared animals can be explained by the initial low rate of milk infusion to allow the stomach to recover from the cannula implantation surgery and the temporary reduction in the rate of milk infusion to help the animals recover from bloat (Tonkiss et al., 1987). In infants receiving enteral feeding, lower feed rates are used after surgery for the same reason. In addition, infants can suffer from a condition similar to bloat called necrotizing entercolitis (Diaz et al., 1980).
Primary dendrites
The 12 days of artificial rearing did not provide a sufficient stimulus to alter the number of primary dendrites. However, we cannot discount the possible alterations in the rest of the dendritic tree. Gazula et al (Gazula et al., 2004) found significant changes within the dendritic tree, including the number of primary dendrites 5 days after spinal cord transection in the rat. However, the spinal cord transection group that performed exercise was not different from the control group (no injury). This suggests that the level of exercise or physical activity affects the development and maintenance of the dendritic tree.
Diameter and Form Factor
The changes in the shape of the soma for hyoglossus motoneurons are consistent with the changes in the shorter diameter. Therefore, it is likely that the differences in form factor are merely reflecting the alterations in shorter diameter. While the increased diameter with advancing age is consistent with previous findings (Smith et al., 2005), the increase in form factor is not. Because form factor was measured and assessed in the same way for both of these studies, it is possible that these findings may be explained by the differences in sample sizes between this study and the previous study.
There was a similar trend for the shorter diameter of styloglossus motoneurons. It seems that the alterations in form factor may be reflecting the changes in the shorter diameter for styloglossus motoneurons as well. Therefore, the artificial rearing process leads to chronic alterations in the shape of the styloglossus motoneurons by retarding the growth of the motoneuron.
Hyoglossus and styloglossus motoneurons reach their adult size by postnatal weeks 2 and 3, respectively (Smith et al., 2005). In the present study, the shorter diameter for hyoglossus motoneurons increased with age for the dam-reared group but not the artificially reared group. Our results are somewhat different from alterations seen with hindlimb suspension models. The shorter diameter of the P14 dam-reared pups were ∼86% of the P60 dam-reared pups while the shorter diameter of the P14 artificially reared pups were ∼99% of their P60 counterparts. Nakano and Katsuta (Nakano and Katsuta, 2000) showed a smaller soma size for motoneurons innervating the soleus in rats using the hindlimb suspension model during postnatal growth. The reason for differences in the magnitude of change between control groups in this study and our study can be explained by the differences between hindlimb suspension and artificial rearing. The hindlimb suspension eliminates all weight bearing activity while the artificial rearing process only eliminates suckling activity. The animals are still able to groom themselves and use their tongues freely. Therefore, the highly coordinated activity of suckling is essential to the normal postnatal development of hyoglossus and styloglossus motoneurons. Previous physiological research supports this notion (Kinirons et al., 2003). This may also be the case for human infants that are necessarily deprived of suckling.
Acknowledgments
The authors would like to thank Mr. Allen Moore for his assistance with the artificial rearing of the animals in this project. We would also like to thank Mr. Vedran Lovic and Dr. Alison S. Fleming at the University of Toronto at Mississauga for their technical assistance with the milk formula.
Grant Sponsor: National Institute of Deafness and Other Communication Disorders
Grant Number: 5 RO1 DC-02008
Literature Cited
- Aldes LD. Subcompartmental organization of the ventral (protrusor) compartment in the hypoglossal nucleus of the rat. J Comp Neurol. 1995;353:89–108. doi: 10.1002/cne.903530109. [DOI] [PubMed] [Google Scholar]
- Diaz J, Samson H, Kessler D, Stamper C, Moore E, Robisch E, Hodson A. Experimental necrotizing enterocolitis: the possible role of bile salts in its etiology and treatment. Pediatr Res. 1980;14:595. [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- Hall WG. Weaning and growth of artificially reared rats. Science. 1975;190:1313–1315. doi: 10.1126/science.1198116. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Beauregard N, Slattery J, Kennedy G. Identification of neonates at risk of developing feeding problems in infancy. Dev Med Child Neurol. 2000;42:235–239. doi: 10.1017/s0012162200000402. [DOI] [PubMed] [Google Scholar]
- Huckstorf BL, Slocum GR, Bain JL, Reiser PM, Sedlak FR, Wong-Riley MT, Riley DA. Effects of hindlimb unloading on neuromuscular development of neonatal rats. Brain Res Dev Brain Res. 2000;119:169–178. doi: 10.1016/s0165-3806(99)00167-4. [DOI] [PubMed] [Google Scholar]
- Jennische M, Sedin G. Speech and language skills in children who required neonatal intensive care. I. Spontaneous speech at 6.5 years of age. Acta Paediatr. 1998;87:654–666. doi: 10.1080/080352598750014076. [DOI] [PubMed] [Google Scholar]
- Jennische M, Sedin G. Speech and language skills in children who required neonatal intensive care. II. Linguistic skills at 6 1/2 years of age. Acta Paediatr. 1999;88:371–383. doi: 10.1080/08035259950169729. [DOI] [PubMed] [Google Scholar]
- Kinirons SA, Shall MS, McClung JR, Goldberg SJ. Effect of artificial rearing on the contractile properties and myosin heavy chain isoforms of developing rat tongue musculature. J Neurophysiol. 2003;90:120–127. doi: 10.1152/jn.00809.2002. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task--reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- McClung JR, Goldberg SJ. Organization of motoneurons in the dorsal hypoglossal nucleus that innervate the retrusor muscles of the tongue in the rat. Anat Rec. 1999;254:222–230. doi: 10.1002/(SICI)1097-0185(19990201)254:2<222::AID-AR8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Messer M, Thoman EB, Galofre A, Dallman T, Dallman PR. Artificial feeding of infant rats by continuous gastric infusion. J Nutr. 1969;98:404–410. doi: 10.1093/jn/98.4.404. [DOI] [PubMed] [Google Scholar]
- Nakano H, Katsuta S. Non-weight-bearing condition arrests the morphological and metabolic changes of rat soleus motoneurons during postnatal growth. Neurosci Lett. 2000;290:145–148. doi: 10.1016/s0304-3940(00)01343-4. [DOI] [PubMed] [Google Scholar]
- Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics. 1999;103:434–439. doi: 10.1542/peds.103.2.434. [DOI] [PubMed] [Google Scholar]
- Smith JC, McClung JR, Goldberg SJ. Postnatal development of hypoglossal motoneurons that innervate the hyoglossus and styloglossus muscles in rat. Anat Rec A Discov Mol Cell Evol Biol. 2005;285A:628–633. doi: 10.1002/ar.a.20204. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Smart JL, Massey RF. Growth and development of rats artificially reared on rats' milk or rats' milk/milk-substitute combinations. Br J Nutr. 1987;57:3–11. doi: 10.1079/bjn19870004. [DOI] [PubMed] [Google Scholar]
- West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]