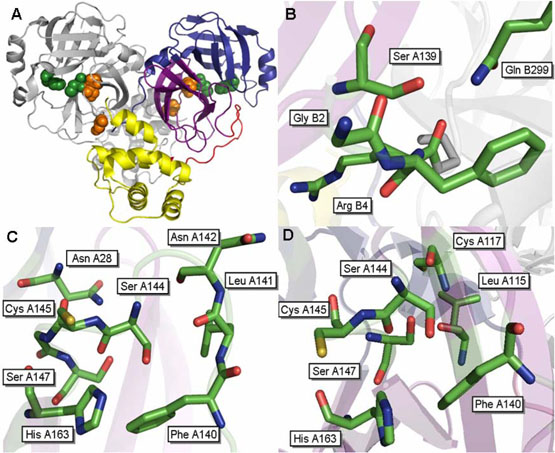
Figure 1.
Structure of the SARS 3CLpro dimer at pH 7.6 [pdb file 1UK3 (18)]. (A) Wild type SARS 3CLpro displayed in ribbon representation. Domain 1 (residues 1-100) is shown in blue, domain 2 (residues 101-183) are colored purple, the loop connecting these two domains to domain 3 is colored red and domain 3 (residues 201-306) is shown in yellow. The catalytic residues, His41 and Cys145, are located in the cleft between the first two domains and are displayed as green spheres. The cluster of serines, Ser139, Ser144 and Ser147 are displayed as orange spheres. Important residues surrounding (B) S139A, (C) S144A and (D) S147A are shown.