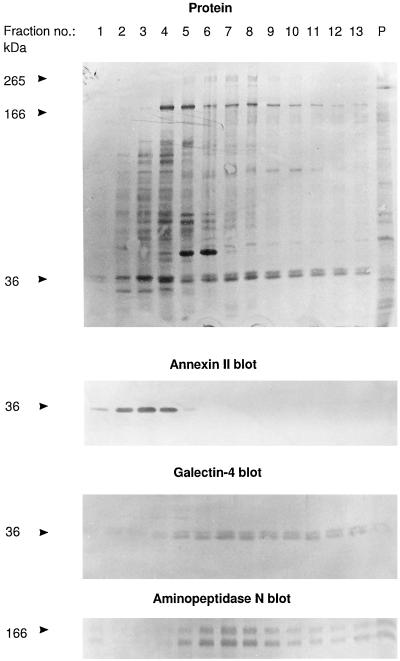
Figure 1.
Identification of galectin-4 as a 36-kDa protein present in high molecular weight clusters. Intestinal mucosa was homogenized in a Potter-Elvehjem homogenizer in ice-cold 25 mM HEPES and 150 mM NaCl, pH 7.0, containing 10 μg/ml aprotinin and 10 μg/ml leupeptin, and centrifuged at 500 × g for 5 min. The supernatant was centrifuged at 48,000 × g for 30 min. The resulting pellet of total membranes was resuspended in the above buffer and solubilized by extraction at 37°C for 10 min with 20 mM CHAPS and 1 mM EDTA. One milliliter of the extract was analyzed by velocity sedimentation in a sucrose gradient as described in MATERIALS AND METHODS. After centrifugation, 0.25 ml of each fraction was mixed with an equal volume of acetone, and after 15 min on ice, protein was pelleted by centrifugation at 20,000 × g for 10 min and analyzed by SDS-PAGE and Western blotting, using the primary antibodies indicated. Lanes 1 and 13 represent the top and bottom fractions of the gradient, respectively, and lane P shows the proteins recovered from the pellet of the centrifugation. Total protein was stained with Coomassie brilliant blue. Both the 166-kDa band of aminopeptidase N and its B-subunit, which is formed by proteolytic cleavage in vivo (Sjöström et al., 1978) are shown at the bottom. Molecular mass values are indicated.