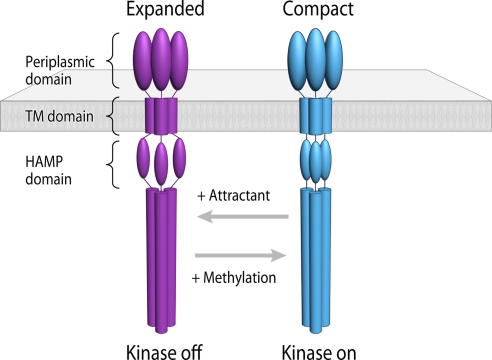
Fig. 6.
A two-state model describing conformational signaling in chemoreceptor trimers. Trimeric chemoreceptors exist in an equilibrium between two conformations and adopt either expanded or compact arrangements of the HAMP signaling domain. Binding of the attractant serine initiates movement in the transmembrane helix (9), which, in turn, shifts the conformational equilibrium of the HAMP domain in favor of the expanded conformation (magenta). Based on the known effects of serine binding to reduce the activity of the CheA kinase, we propose that this expanded conformation of the HAMP domain corresponds to the “kinase-off” state. Conversely, an increase in chemoreceptor methylation shifts the equilibrium in favor of the compact HAMP conformation (cyan), corresponding to the “kinase-on” state.