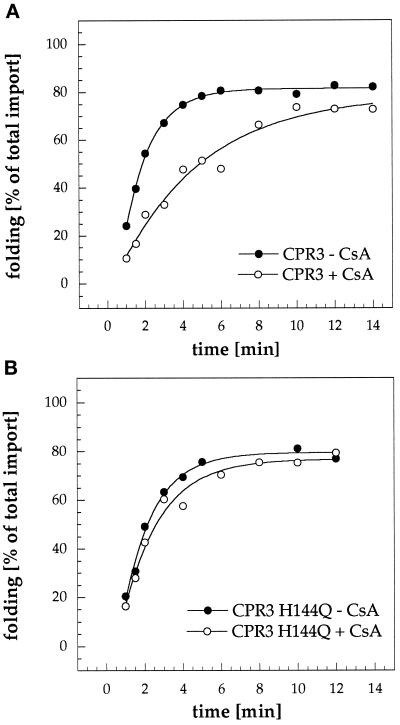
Figure 1.
Cpr3 active-site mutant catalyzes protein refolding in mitochondria. Refolding kinetics of Su9-DHFR imported into mitochondria purified from a cpr3 mutant strain expressing the wild-type CPR3 protein (A) or the CPR3 H144Q prolyl isomerase inactive mutant protein (B). Su9-DHFR was accumulated as the unfolded ATP-depletion intermediate in the mitochondrial intermembrane space. After reisolation, mitochondria were incubated with (+ CsA) or without (− CsA) 2.5 μg/ml CsA. Su9-DHFR was subsequently chased into the matrix by addition of chase buffer containing 2 mM ATP and 5 mM α-ketoglutarate. At the indicated times, samples were withdrawn and treated with Triton X-100 and proteinase K, and the amount of folded protease-resistant DHFR was analyzed by SDS-PAGE and fluorography (for details, see MATERIALS AND METHODS).