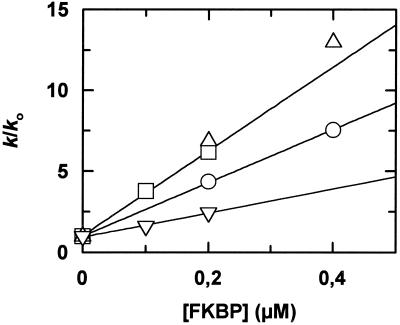
Figure 4.
Wild-type and FKBP12 mutants catalyze ribonuclease T1 refolding. The kinetics of refolding of 0.3 μM RCM-(S54G/P55N)-RNase T1 were measured in the absence and in the presence of increasing concentrations of the wild-type and the mutant forms of FKBP12. The measured catalytic activities in folding are shown as a function of the concentration of wild-type FKBP12 (□), the F106Y (○), the D44V (▵), and the F43Y (▿) mutants. The activities are shown as k/k0, where k and k0 are the rate constants of folding of RCM-(S54G/P55N)-RNase T1 in the presence and absence of the various FKBP12 proteins, respectively. The folding kinetics were measured at 15°C in 0.1 M Tris-HCl and 2.0 M NaCl by the increase in fluorescence at 320 nm.