Abstract
The functional role of IL-12 and IL-23 in host defense and disease following viral infection of the CNS was determined. Instillation of mouse hepatitis virus (MHV, a positive-strand RNA virus) into the CNS of mice results in acute encephalitis followed by a chronic immune-mediated demyelinating disease. Antibody-mediated blocking of either IL-23 (anti-IL-23p19) or IL-12 and IL-23 (anti-IL-12/23p40) signaling did not mute T-cell trafficking into the CNS or antiviral effector responses and mice were able to control viral replication within the brain. Therapeutic administration of either anti-IL-23p19 or anti-IL-12/23p40 to mice with viral-induced demyelination did not attenuate T-cell or macrophage infiltration into the CNS nor improve clinical disease or diminish white matter damage. In contrast, treatment of mice with anti-IL-12/23p40 or anti-IL-23p19 resulted in inhibition of the autoimmune model of demyelination, experimental autoimmune encephalomyelitis (EAE). These data indicate that (1) IL-12 and IL-23 signaling are dispensable in generating a protective T-cell response following CNS infection with MHV, and (2) IL-12 and IL-23 do not contribute to demyelination in a model independent of autoimmune T-cell–mediated pathology. Therefore, therapeutic targeting of IL-12 and/or IL-23 for the treatment of autoimmune diseases may offer unique advantages by reducing disease severity without muting protective responses following viral infection.
Introduction
Interleukin (IL-23) and IL-12 are heterodimeric proteins that exhibit many similar structural as well as functional properties (45). Both IL-23 (p19/p40) and IL-12 (p35/p40) share an identical p40 subunit, and receptors for IL-23 and IL-12 utilize the common IL-12Rβ1 chain (46). Moreover, signaling by these cytokines often elicits similar and overlapping immune responses (45,46). For example, both IL-12 and IL-23 are considered important in amplifying T-cell responses including proliferation and cytokine secretion following specific antigenic challenge (8,27,31,33,41,53,65). Expression of IL-12 is associated with the development of Th-1 responses characterized by IFN-γ secretion from antigen-specific T lymphocytes in response to infection with intracellular pathogens such as viruses, suggesting an important role in host defense. Experimental infection of mice deficient in either IL-12 or IFN-γ signaling with pathogens including herpes simplex virus, murine cytomegalovirus, respiratory syncytial virus, and measles virus results in increased susceptibility to disease, highlighting the importance of the IL-12 and/or IFN-γ signaling axis in antiviral defense in these animal models of disease (3,13,22). However, humans deficient in their ability to either produce or respond to IFN-γ as a result of mutations in IFN-γ signaling receptor, IL-12p40 subunit, or IL-12 receptor reveal increased vulnerability to select intracellular pathogens including mycobacterium and Salmonella, yet display susceptibility to only a limited number of RNA or DNA viruses (9,10,37,44). These findings indicate a more important role for IL-12 and IFN-γ in defense following viral infection of mice, while these factors may be dispensable in generating a protective antiviral response in humans. Expression of IL-23 correlates with the development and expansion of Th-17 cells, and is often associated with mouse models of autoimmune inflammatory diseases including experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (5,8,42,50). Recent studies have revealed potentially important roles for IL-23 in host defense in response to infectious agents, suggesting protection can occur in an IL-12–independent manner that may be related to IL-23 signaling (18,20,35,63). However, the functional significance of IL-23 signaling in response to viral infection is not well defined and highlights paucity in our understanding of how IL-23 may tailor specific immune responses resulting in virus-specific T lymphocytes essential in effective host defense.
Intracranial infection of mice with mouse hepatitis virus (MHV) results in an acute encephalomyelitis followed by a chronic demyelinating disease (1,39). The acute stage of disease is characterized by viral infection of glial cells and widespread growth throughout the parenchyma. Virus-specific CD4+ and CD8+ T cells are generated in draining cervical lymph nodes (dCLN) and traffic into the CNS where the viral burden is reduced (40). However, virus persists within the CNS, primarily in white matter tracts, and animals will often develop an immune-mediated demyelinating disease with pathology similar to the human demyelinating disease multiple sclerosis (MS). Both T cells and macrophages are important in amplifying the severity of myelin damage in mice persistently infected with MHV (60–62). Indeed, blocking expression of the CXC chemokine ligand 10, CXCL10, or CC chemokine ligand 5, CCL5, in mice persistently infected with MHV and undergoing chronic demyelination results in a marked improvement in clinical disease that is associated with reduced accumulation of T cells and macrophages within the CNS and limited spread of demyelination (15,36). Unlike Theiler's murine encephalomyelitis virus, in which autoreactive T cells specific for myelin antigens are elicited via epitope spreading and participate in the pathogenesis of disease, the generation of T cells reactive to myelin epitope(s) during chronic disease in MHV-infected mice is limited and not thought to substantially contribute to demyelination (7,24). While adoptive transfer of T cells from MHV-infected rats to naãve recipients results in inflammatory lesions (59), no evidence of a similar response in mice has been reported.
A protective immune response to MHV infection during acute disease is characteristic of a Th-1 response and is associated with robust IFN-γ secretion and cytolytic activity by virus-specific T cells (2,48,55). However, the signaling mechanisms responsible for eliciting IFN-γ–secreting T cells remain elusive. For example, infection of mice deficient in either the IL-12p40 or p35 chain (IL-12–deficient mice) with hepatotropic strains of MHV resulted in a robust Th-1 response characterized by high IFN-γ levels and muted secretion of IL-4 (51). These findings suggest that alternative pathways exist in which IFN-γ production by virus-specific T cells occurs independently of IL-12 expression. Therefore, the present study was undertaken to further evaluate how IL-12 and/or IL-23 signaling contributes to host defense in response to MHV infection of the CNS. Through use of antibodies specific for either IL-23 (anti-IL-23p19) or reactive to both IL-12/23 (anti-IL-12/23p40), data are presented that support and extend previous findings demonstrating an important role for IL-23 in inflammatory autoimmune demyelinating disease, as treatment with either anti-IL-23p19 or anti-IL-12/23p40 reduced clinical disease severity in mice immunized against myelin basic protein (MBP). However, blocking IL-23 and/or IL-12 signaling did not affect the generation or trafficking of MHV-specific CD4+ or CD8+ T cells into the CNS following infection, nor alter viral clearance or reduce the severity of white matter damage when compared to control mice. Therefore, these data demonstrate that protective immune responses to MHV infection occur independently of either IL-12 or IL-23, suggesting that alternative mechanisms exist by which virus-specific T cells are generated. Furthermore, these findings highlight that muting IL-23 signaling does not dampen the ability to generate a protective cellular immune response following infection of mice with MHV.
Materials and Methods
Antibodies
A neutralizing rat monoclonal antibody to mouse IL-23p19 (CNTO 209) was generated by immunizations with a mixture of DNA and protein immunogens. For mouse IL-23 protein production, individual mammalian expression plasmids for mouse p40 and p19 subunits were mixed at a 2:1 mass ratio for p19:p40, and used to transiently transfect HEK 293E cells. To aid with purification, a His tag was placed at the C-terminus of the p19 protein and following transfection, conditioned medium was harvested and standard immobilized metal affinity chromatography utilized to isolate the His containing heterodimeric IL-23 away from contaminating proteins including free p40. The purified fraction was then concentrated and dialyzed into PBS. RT-PCR was used to amplify mouse IL-23 p19 cDNA, which was then inserted into a mammalian expression vector containing the human cytomegalovirus immediate early promoter. Sprague-Dawley rats were immunized with two successive intradermal administrations of plasmid IL-23 p19 DNA in both ears on days 0 and 14 (15 μg/ear). The rats then received three subsequent subcutaneous injections of recombinant mouse IL-23 protein (50 μg on day 28, 40 μg on day 85, and 15 μg on day 343), followed by a subcutaneous injection with Titermax adjuvant (Sigma, St. Louis, MO) (15 μg on day 375), and a simultaneous intradermal administration without Titermax (15 μg on day 375). A final booster injection of 20 μg of protein was administered IV 4 d prior to harvest (day 377). Fusion of rat splenocytes to mouse myeloma cells was performed by conventional hybridoma techniques and standard EIA methods were used to identify clones secreting IL-23–specific antibodies. Positive clones were subcloned twice by limiting dilution. Negative control rat IgG was purchased (Jackson Immuno Research, West Grove, PA). Negative control mouse IgG (CNTO 1322) was generated by Centocor. Neutralizing rat anti-mouse IL-12/23p40 (C17.8) and rat anti-mouse IL-12p35 (C18.2) were generous gifts of Dr. Giorgio Trinchieri and the Wistar Institute (Philadelphia, PA). Ascites was generated at Harlan Bio-products (Indianapolis, IN) and antibodies were purified by protein G affinity chromatography. CNTO 3913 is a chimeric antibody constructed by Centocor using variable regions from a neutralizing rat anti-mouse IL-12/23p40 mAb generated at Centocor through immunization of Sprague-Dawley rats with recombinant mouse IL-12 (R&D Systems, Minneapolis, MN, USA) in adjuvant, followed by standard hybridoma cell fusion methods. Functional variable region genes were identified from the hybridoma cell line, and the heavy chain and light chain variable region genes were cloned into murine IgG2a and kappa expression vectors, respectively, to generate a chimeric rat/mouse anti-IL12/23p40 mAb (CNTO 3913).
IL-12 and IL-23 ELISA
Mouse IL-12, IL-23, and p40 (0.5 μg/mL) were coated overnight on Nunc Maxisorp plates in PBS. After the plates were washed and blocked, anti-IL-23p19 antibody (CNTO 209, Centocor) was titrated and allowed to bind for 2 h. Bound protein was detected using 1:5000 HRP-conjugated donkey anti-rat IgG antibody (Jackson Immuno Research) followed by substrate.
IL-12 and IL-23 neutralization
Single-cell suspensions were prepared from spleens of C57BL/6 mice, and 2.5 × 106 cells/mL were cultured in complete RPMI with 10 U/mL rhIL-2 (PeproTech, Rocky Hill, NJ, USA) and 1 ng/mL mouse IL-23 or IL-12 (R&D Systems), either alone or pre-incubated with anti-IL-23p19 (CNTO 209), anti-IL-12p35 (C18.2), or anti-IL-12/23p40 (C17.8 or CNTO 3913) antibodies. Rat IgG (Jackson ImmunoLabs) and mouse IgG (CNTO 1322, Centocor) were also used as negative controls. Cell cultures were incubated for 3 d. Supernatants were collected and analyzed for IL-17 or IFN-γ protein by ELISA (R&D Systems) per the manufacturer's instructions.
EAE analysis and mice
Female B10.PL mice (Jackson Laboratories, Bar Harbor, ME) from 6–8 wk of age were used. Mice were injected SC over four sites on the back with a total of 100 μL of complete Freund's adjuvant (CFA) combined with 200 μg guinea pig-myelin basic protein (Sigma). Mice also received 200 ng pertussis toxin (List Biological, Campbell, CA) IP in 0.2 mL PBS at the time of immunization and 48 h later. Mice received three once weekly IP injections of either PBS or 20 mg/kg anti-IL-12/23p40 (C17.8), anti-IL-23p19 (CNTO 209), or rat IgG (Jackson ImmunoLabs) antibodies starting the day prior to EAE induction. Animals that demonstrated clinical signs were scored as follows: limp tail or waddling gait with tail tonicity = 1; waddling gait with limp tail (ataxia) = 2; ataxia with partial limb paralysis = 2.5; full paralysis of one limb = 3; full paralysis of one limb with partial paralysis of second limb = 3.5; full paralysis of two limbs = 4; moribund = 4.5; and death = 5. EAE incidence, median time to onset, disease burden over time, highest clinical signs during acute EAE, number of EAE relapses, and severity of EAE relapses ± SEM are described. Relapses were defined by a full point drop in clinical sign score sustained for at least 2 observed days, followed by a full point increase in clinical sign score sustained for at least 2 observed days. Scores for animals that were sacrificed or scored a 5 were not included in the mean daily analysis of clinical signs for the remainder of the experiment. Incidence of EAE and mortality were compared between groups using Fisher's exact test. Time to EAE onset was evaluated using life table methods and the log-rank test was used for comparisons between groups. The number of relapses and highest clinical scores during acute and relapse phases were analyzed using the Cochran-Mantel-Haenszel ANOVA statistic. These analyses were conducted using the SAS System for Windows, V8 (SAS Software, Cary, NC). The weighted mean clinical score was calculated for individual animals to determine the disease burden over time. This area-under-the-curve type analysis determines the average daily disease burden for each animal over the entire study. The units of this end-point are the same as the units of an individual daily clinical score. Disease burden over time was analyzed via a standard linear model and analysis of variance (ANOVA). Pairwise comparisons among the groups were evaluated. Disease burden over time analyses were performed using the R software environment (a). For all analyses, p values <0.05 were considered significant. No adjustments were made for multiple testing.
Reverse transcriptase-PCR
RNA was extracted from the brains of MHV-infected and sham-infected mice at defined times post-infection (PI) using TRIzol® Reagent (Invitrogen, Carlsbad, CA), treated with RQ1 RNase-free DNase (Fisher Scientific, Pittsburgh, PA), and purified by phenol/chloroform extraction. cDNA was generated using an MMLV reverse transcriptase (RT) kit (Invitrogen) and random hexamer primers (Promega, Madison, WI). PCR was performed on the resulting cDNA with specific primers for GAPDH (forward, 5′-ACTCACGGCAAATTCAACG; reverse, 5′-CCCTGTTGCTGTAGCCGTA), IL-23p19 (forward, 5′-CATGGGGCTATCAGGGAGTA; reverse, 5′-AATAATGTGCCCCGTATCCA), and IL-12p35 (forward, 5′-GACTTGAAGATGTACCAGACAG; reverse, 5′-GAGATGAGATGTGATGGGAG). Amplification was performed on an Eppendorf MasterCycler (Westbury, NY) using the following profile: step 1, denaturation at 94°C for 45 sec; step 2, annealing at 60°C for 45 sec; and step 3, extension at 72°C for 1 min. Steps 1–3 were repeated 34 times for a total of 35 cycles and were followed by a 7-min incubation at 72°C. Sequence analysis of GAPDH, IL-23p19, and IL-12p35 amplicons confirmed primer specificity (54).
Virus and mice
MHV strain J2.2V-1 was used for the experiments described (58). Age-matched 5- to 7-week-old C57BL/6 mice (H-2b background) were purchased from the National Cancer Institute, Bethesda, Maryland, and used for MHV experiments. Following anesthetization by IP injection with ketamine, mice were injected intracranially (IC) with virus (500 PFU) suspended in 30 μL of sterile saline. Experimental groups of mice infected with MHV were injected IP with either purified anti-IL-23p19 (CNTO 209, 500 μg/dose), anti-IL-12/23p40 (CNTO 3913, 500 μg/dose), or mouse IgG isotype control (CNTO 1322, 500 μg/dose) according to the following experimental schedules. For the acute MHV infection studies, mice were injected on days 0 and 7 PI with anti-IL-23p19 or control antibodies, and mice receiving anti-IL-12/23p40 or control antibodies were injected on days 0, 4, 8, and 12 PI. For the chronic MHV infection studies, mice were injected on days 14 and 21 PI with anti-IL-23p19 or control antibodies, and mice receiving anti-IL-12/23p40 or control antibodies were injected on days 12, 16, and 20 PI. Animals were sacrificed at defined time points and tissues removed for analysis. One-half of each brain at each time point was used for plaque assay on a mouse astrocytoma cell line (30). Experiments for all animal studies described have been reviewed and approved by an appropriate institutional review committee.
Flow cytometry
Mononuclear cells were obtained from half-brains and dCLN (deep and superficial nodes) at defined times post IC infection with MHV using previously described methods (23,30). Immunophenotyping of cells was performed using allophycoerythrin (APC)-conjugated rat anti-mouse CD4 and CD8 (Pharmingen, San Diego, CA), FITC-conjugated rat anti-mouse F4/80 (Serotec, Oxford, U.K.), and APC-conjugated rat anti-mouse CD45 (Pharmingen) (23). In all cases, isotype-matched control antibodies were used. Virus-specific CD4+ and CD8+ T cells recognizing their respective immunodominant epitope between amino acids 133 and 147 of the membrane (M) glycoprotein (M133–147) and surface (S) glycoprotein (S510–518) were determined by intracellular IFN-γ staining using previously described methods (14,16). In brief, 1 × 106 total cells were stimulated with 5 μM final concentration of viral peptides for 6 h at 37°C in media containing Golgi stop (Cytofix/Cytoperm kit; Pharmingen), after which cells were washed and blocked with PBS containing 10% FBS and a 1:200 dilution of rat anti-mouse CD 16/32 antibody CD16/32 (BD Pharmingen). Cells were then stained for surface antigens using APC-conjugated rat anti-mouse CD4 and CD8 (BD Pharmingen), according to the viral peptide stimulation condition, for 45 min at 4°C. Cells were fixed and permeabilized using a Cytofix/Cytoperm kit and stained for intracellular IFN-γ using phycoerythrin (PE)-conjugated anti-IFN-γ (1:50; XMG1.2, BD Pharmingen) for 45 min at 4°C. Cells were washed and flow cytometry was performed using a FACStar flow cytometer (Becton Dickinson, Mountain View, CA). Frequency data are presented as the percentage of positive cells within the gated population. Total cell numbers were calculated by multiplying these values by the total number of live cells isolated.
Cytokine analysis
Supernatants from brain homogenates were analyzed for cytokine expression in a multiplexed fashion using the cytokine 20-plex AB bead kit (BioSource Invitrogen, Carlsbad, California). This kit comprises analyte-specific components for the measurement of mouse FGF basic, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p40/p70, IL-13, IL-17, CXCL10, CXCL1, CCL2, CXCL9, CCL3, TNF-α, and VEGF. Samples were run in duplicate in accordance with the manufacturer's directions. Samples were analyzed using a Luminex 100 IS System (Austin, TX) running Star Station 2.0 software (Applied Cytometry Systems, Sacramento, CA). IL-23p19 was quantified using standard ELISA methods with rat anti-mouse IL-23p19 (CNTO 209) capture antibody, biotinlyated rat anti-mouse IL-12/23p40 (C17.8) antibody, and mIL-23 standard (R&D Systems).
Histology
Spinal cords were fixed in normal balance formalin for 24 h and then embedded in paraffin (30). Sections of spinal cords were stained with luxol fast blue to identify myelin (blue) and counterstained with Harrison hematoxylin and eosin to visualize cellular inflammation. Slides containing stained spinal cord sections were blinded and the severity of demyelination assessed using a light microscope. Demyelination was scored as follows: 0 = no demyelination; 1 = mild inflammation accompanied by loss of myelin integrity; 2 = moderate inflammation with increasing myelin damage; 3 = numerous inflammatory lesions accompanied by significant increase in myelin stripping; and 4 = intense areas of inflammation accompanied by numerous areas of phagocytic cells engulfing myelin debris (24,29,30).
Statistical analysis of MHV studies
Statistically significant differences between antibody treatment groups were determined by Mann-Whitney rank sum test. Statistical analyses were done using Sigma-Stat 2.0 software (Jandel, Chicago, IL) and p values of ≤0.05 were considered significant.
Results
Anti-IL-23p19 mAb binds mouse IL-23 and neutralizes bioactivity
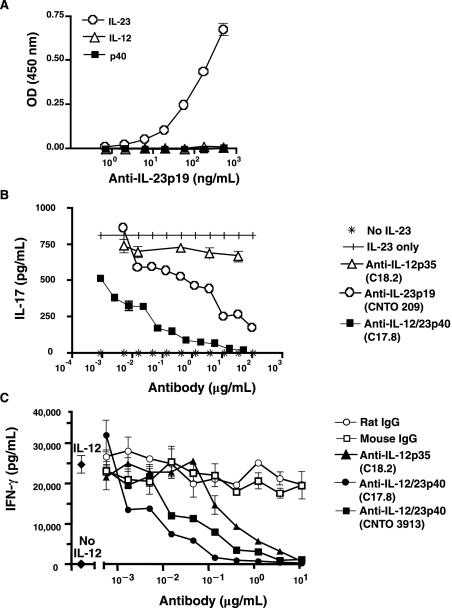
A monoclonal antibody specific for mouse IL-23p19 was developed as described in the materials and methods section. Mouse IL-12, IL-23, or p40 (shared by both IL-23 and IL-12) were coated on ELISA plates and anti-IL-23p19 binding determined. As shown in Fig. 1A, anti-IL-23p19 recognizes IL-23, but not either IL-12 or the p40 subunit, in a concentration-dependent manner indicating that this antibody is specific for mouse IL-23. To test the ability of anti-IL-23p19 to neutralize signaling, mouse splenocyte cultures were incubated with recombinant mouse IL-23 and IL-2, and IL-17A production determined. Inclusion of either anti-IL-23p19 or anti-IL-12/23p40 mAbs resulted in reduced IL-17A secretion by splenocytes and was sensitive to antibody concentration (Fig. 1B). Of note, CNTO 3913 and C17.8 anti-IL-12/23p40 mAbs demonstrated comparable neutralization potency against mouse IL-23 in this assay (data not shown). Incubation of cells with anti-IL-12p35 did not result in an appreciable decrease in IL-17A production. These data demonstrate that anti-IL-23p19 is specific for IL-23 and blocks signaling. In addition, the ability of anti-IL-12/23p40 blocking antibody to neutralize signaling was also determined. As shown in Fig. 1C, inclusion of either anti-IL-12p35 or anti-IL-12/23p40 antibodies resulted in a dose-dependent reduction in IFN-γ secretion by splenocytes incubated with recombinant IL-12. These data confirm that anti-IL-12/23p40 is capable of inhibiting IL-12–induced IFN-γ secretion by T cells. As expected, since the anti-IL-23p19 antibody does not bind mouse IL-12 (Fig. 1A), there was no neutralization of mouse IL-12 when anti-IL-23p19 was tested in this assay (data not shown).
FIG. 1.
Anti-IL-23 antibody is specific for mouse IL-23 and neutralizes IL-23 bioactivity. (A) Mouse IL-12, IL-23, or p40 homodimer were coated on ELISA plates and anti-IL-23 antibody was titrated. Data are shown as the mean optical density (OD) of replicate wells ± SD and are representative of two experiments. IL-17 or IFN-γ production was measured in supernatants from C57BL/6 splenocyte cultures supplemented with recombinant mouse (B) IL-23 or (C) IL-12 and dilutions of anti-IL-12/23p40, anti-IL-12p35, or anti-IL-23p19 antibodies. Data are shown as the mean of replicate wells ± SEM and are representative of three experiments.
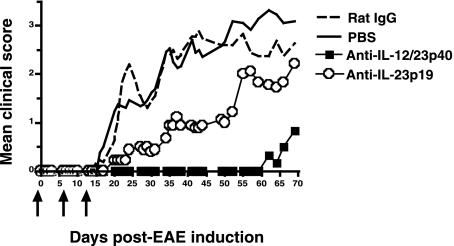
We next determined the in vivo blocking efficacy of anti-IL-23p19 and anti-IL-12/23p40 antibodies. EAE is an autoimmune demyelinating disease that shares many clinical and histologic similarities with the human autoimmune demyelinating disease MS (5,12). Previous studies have demonstrated that IL-23p19–deficient mice are resistant to EAE and treatment of mice with a neutralizing anti-IL-23p19 antibody reduces disease severity (8). Blocking IL-23 signaling effectively limits specific pathways required for CNS autoimmune inflammation by inhibiting the generation of IL-17–producing T cells. To test the efficacy of the anti-IL-23p19-specific antibody for in vivo neutralization, chronic-relapsing EAE was induced in B10.PL mice by injecting MBP as described in the materials and methods section. Mice were treated with either anti-IL-23p19 or anti-IL-12/23p40 mAbs, control rat IgG, or PBS, and clinical disease progression in experimental groups was determined 70 d post-MBP immunization. Initiation of treatment with anti-IL-12/23p40 was more effective at suppressing mean daily clinical sign scores (Fig. 2), EAE incidence, the highest clinical score during acute EAE, and relapse number and severity (Table 1), when compared to anti-IL-23p19 treatment. However, both antibodies were able to significantly reduce mean daily clinical sign scores (Fig. 2), median time to onset, and disease burden over time when compared to mice treated with control antibody or PBS (Table 1).Anti-IL-23p19 and anti-IL-12/23p40 treatment also suppressed EAE severity when dosing was initiated on days 10 or 30 after EAE induction (data not shown). Collectively, these data demonstrate that both anti-IL-23p19 and anti-IL-12/23p40 antibodies are capable of specifically blocking cytokine signaling in vivo and reducing the severity of clinical disease associated with autoimmune-mediated neuroinflammation and demyelination.
FIG. 2.
IL-23 neutralizations inhibit MBP-induced EAE in B10.PL mice. EAE was induced in B10.PL mice as described in the materials and methods section, and animals were treated with PBS, negative control rat IgG, anti-IL-12/23p40, or anti-IL-23p19 mAbs at days −1, 6, and 13 (arrows). Daily clinical scores were averaged for the group and are shown through 70 d post–EAE immunization.
Table 1.
Administration of Anti-IL-23p19 and Anti-IL-12/23p40 Reduces EAE Clinical Disease
| Treatment regimen | Incidence | Median time to onset (days) | Disease burden over time (mean ± SEM) | Highest acute clinical sign (mean ± SEM) | No. of relapses (mean ± SEM) | Relapse severity (mean ± SEM) |
|---|---|---|---|---|---|---|
| Day −1 (start) | ||||||
| PBS | 13/13 (100%) | 22.5 | 1.66 ± 0.20 | 3.6 ± 0.3 | 0.7 ± 0.2 | 3.8 ± 0.1 |
| Rat IgG | 11/11 (100%) | 21.5 | 1.27 ± 0.20 | 3.8 ± 0.4 | 0.5 ± 0.2 | 3.5 ± 0.3 |
| Anti-IL-12/23p40 | 3/6 (50%)a,b | >69a,b | 0.18 ± 0.24a,b | 0.8 ± 0.5a,b | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Anti-IL-23p19 | 8/9 (89%) | 51.0a,b | 0.80 ± 0.24a | 2.8 ± 0.5 | 0.4 ± 0.2 | 3.3 ± 0.3 |
Significantly different from PBS at p < 0.05.
Significantly different from rat IgG at p < 0.05.
IL-12 and IL-23 are not required for viral clearance from the brain
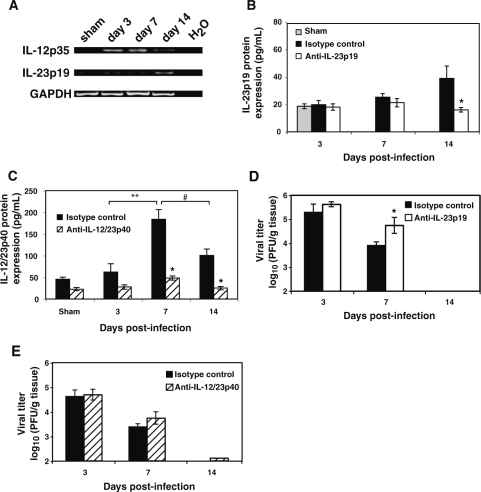
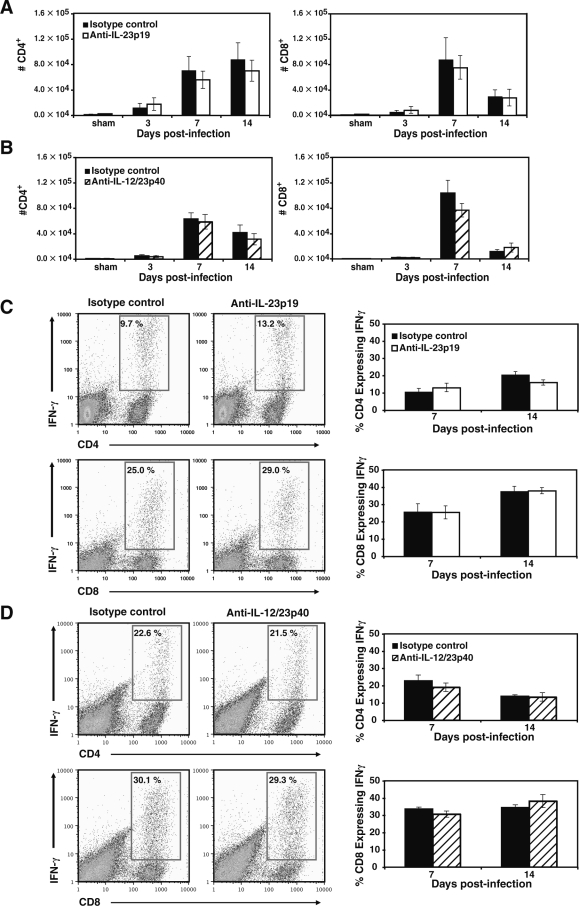
Utilizing primers specific for either IL-23p19 or IL-12p35, we tested for mRNA expression within the brain following IC MHV infection of the CNS (Fig. 3A). Expression of IL-12p35 was not detectable within the brains of sham-infected mice, but was clearly present at days 3, 7, and 14 PI. Transcripts for IL-23p19 are present in the brains of control mice and MHV infection results in an apparent increase in transcript levels at later times PI. Increased mRNA transcripts correlated with elevated levels of IL-23p19 and IL-12/23p40 (Fig. 3B and C) within the brains of infected mice as determined by ELISA, although IL-23p19 was present at markedly reduced levels compared to levels of IL-12/23p40 between 7 and 14 days PI. Levels of IL-12/23p40 were significantly elevated within the brains of mice at day 7 PI when compared to either day 3 (p ≤ 0.007) or day 14 (p ≤ 0.03) PI (Fig. 3C). We next tested whether blocking either IL-12 or IL-23 affected antiviral immune responses following MHV infection of the CNS. C57BL/6 mice were infected IC with MHV and treated IP with either anti-IL-23p19 or anti-IL-12/23p40 mAbs or control IgG, and viral clearance from the brains determined. In vivo treatment with neutralizing anti-IL-23p19 or anti-IL-12/23p40 antibodies significantly reduced their respective cytokine protein levels in the brains of MHV-infected mice, compared to control treatment groups (Fig. 3B and C). Viral titers were elevated (p ≤ 0.05) within the brains of mice treated with anti-IL-23p19 (Fig. 3D) at day 7 PI compared to mice treated with the isotype control antibody, suggesting an impaired ability to control viral replication. However, there were no differences in viral titers within the brains of mice treated with anti-IL-12/23p40 (Fig. 3E), compared to mice treated with control antibody at any time point examined. Ultimately, both groups of mice reduced viral titers to levels comparable to control mice at day 14 PI (∼100 PFU/g tissue). These data indicate that neither IL-12 nor IL-23 signaling is required for viral clearance from the brains of MHV-infected mice. In order to further evaluate host defense to MHV infection, we used flow cytometric phenotyping to determine T-cell responses in mice treated with either anti-IL-23p19 or anti-IL-12/23p40 blocking antibody. There were no significant differences in total CD4+ or CD8+ T-cell accumulation in the brains of mice treated with either anti-IL-23p19 (Fig. 4A) or anti-12/23p40 (Fig. 4B) at any time point examined. Moreover, there were no differences in frequencies of virus-specific IFN-γ–producing CD4+ or CD8+ T cells (as determined by intracellular IFN-γ staining in response to defined immunogenic viral peptides) within the brains of MHV-infected mice in which either IL-23 (Fig. 4C) or IL-12/23 (Fig. 4D) signaling was blocked when compared to control-treated mice at days 7 and 14 PI.
FIG. 3.
Antibody blocking of either IL-23p19 or IL-12/23p40 does not affect the ability to control viral replication within the brain. (A) RT-PCR revealed that intracranial instillation of MHV results in detection of transcripts specific for IL-23p19 and IL-12p35 in the brain at the indicated times post-infection. Each lane is from the brain of a single mouse and is representative of n = 2–3 for each time point. ELISA indicating that both IL-23p19 (B) and IL-12/23p40 (C) are detected within the brains of MHV-infected mice at the defined time points post-infection (n = 3–5 mice for each time point). Levels of IL-12/23p40 within the isotype control-treated mice were significantly elevated within the brain at day 7 post-infection when compared to levels present at day 3 (**p ≤ 0.007) and day 14 (#p ≤ 0.03) post-infection. Neutralizing antibody treatment significantly reduced (p ≤ 0.05) cytokine protein levels during peak expression in the brain compared to isotype control treatments at the indicated times post-infection. Treatment of MHV-infected mice with neutralizing antibodies specific for either IL-23p19 (D) or IL-12/23p40 (E) does not impair control of viral replication (PFU/g tissue) from the CNS. Data are presented as average ± SEM and represent a minimum of two independent experiments with a total n = 5–9 for each antibody treatment group per time point examined. *p ≤ 0.05 compared to mice treated with control antibody.
FIG. 4.
Blocking IL-12/23 does not alter T-cell infiltration into the CNS of MHV-infected mice. MHV-infected mice were treated with either anti-IL-23p19 or anti-12/23p40 neutralizing antibodies and the effects on T-cell infiltration into the CNS evaluated at specific time points post-infection. Blocking either IL-23 (A) or IL-12/23 (B) did not reduce total numbers of CD4+ or CD8+ T cells entering the CNS when compared to mice treated with control antibody. Similarly, treatment with anti-IL-23p19 (C) or anti-IL-12/23p40 (D) did not alter infiltration of virus-specific CD4+ or CD8+ T cells compared to control-antibody-treated mice, as shown in representative FACS dot-plots and corresponding bar graphs. The bar graphs portray the frequency of virus-specific CD4+ or CD8+ T cells within their respective T-cell subset on days 7 and 14 post-infection. The frequencies of virus-specific T cells were derived from dual-positive IFN-γ+ CD4+/CD8+ populations as shown in boxed regions of representative dot-plots from day 7 post-infection. Data are presented as average ± SEM and are representative of two independent experiments with a total n = 4–8 for each antibody treatment group per time point examined.
Antiviral T-cell effector functions and IL-23 signaling
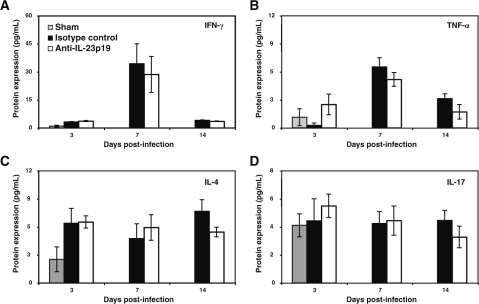
Infiltrating virus-specific T cells eliminate MHV from the brain through secretion of IFN-γ by both CD4+ and CD8+ T cells and cytolytic activity by CD8+ T cells (2,48,55). Although viral clearance from the brains of mice treated with either anti-IL-23p19 or IL-12/23p40 was not affected, treatment with anti-IL-23p19 antibody did result in significantly higher viral titers at day 7 PI compared to mice treated with control antibody, suggesting muted antiviral functional activity by infiltrating T cells. Therefore, to determine if antibody treatment affected antiviral effector functions associated with virus-specific T cells, cytokine profiles within the brains of experimental mice were determined by ELISA. There was no difference in expression of IFN-γ within the brains of mice treated with anti-IL-23p19 compared to animals treated with control antibody at any time point examined (Fig. 5A). On day 3 PI the levels of IFN-γ secretion in the brains of MHV-infected mice were slightly increased compared to sham-infected mice. However, a dramatic increase in IFN-γ secretion (∼30 pg/mL in experimental mice) was observed in both infected treatment groups on day 7 PI, correlating with infiltration of virus-specific T cells into the CNS (39). By day 14, viral titers in the brain were reduced and IFN-γ levels in anti-IL-23p19 and control antibody-treated mice decreased appreciably (Fig. 5A). Expression profiles of TNF-α at days 3, 7, and 14 follow a similar trajectory as IFN-γ between experimental groups (Fig. 5B). However, at day 3 PI, TNF-α levels were elevated within the brains of anti-IL-23p19–treated mice compared to control-antibody-treated mice, yet this difference was not significant. However, there were no differences in TNF-α levels at either days 7 or 14 between anti-IL-23p19–treated or control mice (Fig. 5B). Notably, TNF-α levels were markedly reduced within the brain compared to IFN-γ levels, supporting earlier studies indicating that MHV infection effectively reduces synthesis of TNF-α protein (56). Blocking IL-23 did not significantly change the expression of IL-4 levels in the brain compared to control-treated mice, although there was a slight reduction on day 14 PI (Fig. 5C). Similarly, MHV infection did not result in increased IL-17 expression in the brain, and blocking IL-23 expression had no effect compared to control mice at any time point examined (Fig. 5D). There were no differences in production of detectable levels of other cytokines or chemokines measured within the brains of mice treated with either anti-IL-23p19 or control antibody (data not shown). Finally, there were no differences in cytolytic activity of CNS-infiltrating CD8+ T cells between anti-IL-23p19– and control antibody–treated mice, indicating that IL-23 does not influence CTL activity in response to MHV infection (data not shown).
FIG. 5.
Anti-IL-23p19 treatment does not modulate cytokine expression in the CNS of MHV-infected mice. The temporal expression of cytokines was determined by ELISA in sham-infected and MHV-infected mice treated with either anti-IL-23p19 or control antibody. Cytokines examined include IFN-γ (A), TNF-α (B), IL-4 (C), and IL-17 (D). Data are presented as average ± SEM and represent two independent experiments with a total n = 4 for each antibody treatment group per time point examined.
Anti-IL-23p19 does not reduce demyelinating disease in persistently infected mice
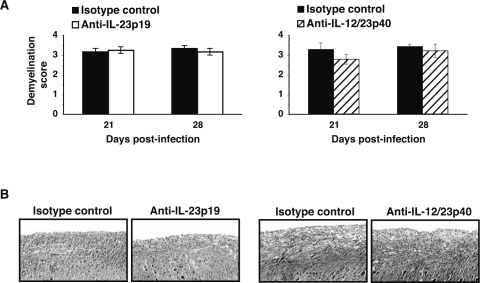
Mice were infected IC with MHV and antibody treatment was initiated between days 12 and 14, which represents a point at which demyelination is established (36). There were no differences in clinical disease progression between mice treated with either anti-IL-12/23p40 or IL-23p19 when compared to mice treated with control antibody (data not shown). Analysis of spinal cords from mice at days 21 and 28 indicated that anti-IL-23p19 or anti-IL-12/23p40 treatment did not reduce the severity of white matter damage or demyelination as compared to control mice (Fig. 6A, B, C, and D). Accordingly, immunophenotyping the composition of the cellular infiltrate within the brains and spinal cords indicated similar numbers and frequency of T cells and macrophages when anti-IL-23p19– or anti-IL-12p40–treated mice were compared to control-treated mice on days 21 and 28 PI (data not shown).
FIG. 6.
Chronic MHV-induced demyelinating disease is not affected following anti-IL-23p19 or anti-IL-12/23p40 treatment. Histologic scoring of the severity of white matter damage to the spinal cords of mice from anti-IL-23p19 (A) and anti-IL-12/23p40 (B) treatment groups indicated no differences in disease severity at either day 21 or 28 post-infection compared to control treated mice. Data were derived from a minimum of three mice per antibody treatment group for each time point, and represent at least two independent experiments. Representative luxol fast blue staining of spinal cords (×100) revealed no difference in the severity of demyelination in anti-IL-23p19 (A) and anti-IL-12/23p40 (B) treated mice when compared to control treated mice at day 21 post-infection. Data are presented as average ± SEM.
Discussion
The present study was undertaken to investigate IL-12 and IL-23 signaling mechanisms associated with a protective immune response following viral infection of the CNS using the MHV model of viral-induced encephalomyelitis. In addition, the functional role of IL-12 and IL-23 in contributing to demyelination in MHV-infected mice was also determined. Previous studies have demonstrated that MHV infection of either IFN-γR−/− mice or IFN-γR−/− mice results in increased mortality accompanied by increased viral titers within infected tissues, supporting the importance of generating a Th-1 response in host defense (17,48,51,52). However, the signaling mechanisms promoting the development of a Th-1 response in MHV-infected mice remain elusive. Parra et al. (47) demonstrated increased expression of numerous cytokines, including IL-12/23p40, within the brains following either lethal or non-lethal coronavirus-induced acute encephalomyelitis, indicating that IL-12 expression may influence the generation of IFN-γ–secreting T cells. However, MHV infection of IL-12–deficient mice (lacking either the p35 or p40 subunit) were able to clear virus from the liver in an IFN-γ–dependent manner, indicating that IL-12 signaling is not essential for protection (51,52). Using antibodies specific for IL-12/23 or IL-23, the work presented here supports previous findings and demonstrates that neither cytokine is necessary for generating functional MHV-specific T cells in response to CNS infection or trafficking of virus-specific T cells into the CNS. In addition, antibody-mediated neutralization of IL-23 had no effect on the secretion of IFN-γ. Although viral titers were eventually reduced below levels of detection in mice treated with blocking IL-23p19 antibody by day 14 PI, there was a significant increase in viral titers within the brains at day 7 PI in anti-IL-23p19–treated mice, suggesting that IL-23 signaling may have some role in controlling viral replication. Clearly, this is not an effect on T-cell antiviral function, as inhibition of IL-23 signaling did not affect either cytokine secretion or CTL activity of infiltrating virus-specific T cells. However, it is possible that IL-23 may influence as of yet undefined antiviral effector responses by resident cells of the CNS that participate in host defense during acute disease. Administration of anti-IL-23p19 or anti-IL-12/23p40 antibody in mice persistently infected with virus did not affect either T-cell or macrophage accumulation in the CNS, or lessen the severity of demyelination, indicating that IL-23 and IL-12 signaling is not important during chronic demyelinating disease in mice persistently infected with MHV. Our data highlight the differences between IL-23 in a model in which autoreactive lymphocytes are not thought to participate in disease (i.e., MHV infection) and EAE, which is characterized by infiltration and accumulation of autoreactive T cells that are essential in driving demyelination. Importantly, we feel that IC infection, while not a natural route of infection, does not invalidate conclusions derived from this study, as this method of infection reproducibly results in an acute encephalomyelitis followed by a chronic demyelinating disease. Moreover, this method of infection results in generation of virus-specific lymphocytes within peripheral lymphatic tissue that occurs following peripheral administration of virus (40).
Instillation of MHV into the CNS resulted in detectable mRNA transcripts specific for IL-12p35 and IL-23p19 within the brain following viral infection. Although the cellular source for these transcripts was not determined, it is likely that both astrocytes and microglia produce IL-12 and IL-23 in response to MHV infection, as previous studies have revealed that these cells are capable of expressing these cytokines both in vivo and in vitro (6,32,34). These findings indicate that genes responsible for encoding IL-12 and IL-23 contain promoter elements sensitive to factors released in response to MHV infection, and that preferential expression of either IL-12 or IL-23 does not occur. This observation is not unique, as both gram-positive and gram-negative bacteria can elicit production of both IL-12 and IL-23 (4). Moreover, specific agonists for toll-like receptors including CpG and LPS also stimulate IL-12 and IL-23 expression (49,57). Although MHV infection promotes expression of IL-12 and IL-23 within the brain as well as in secondary lymphatic tissue (data not shown), a distinct Th-1 lineage is developed, as IFN-γ is a predominant cytokine expressed by virus-specific T cells, and Th-2 cytokines such as IL-4 are present at comparatively lower levels within both brain and dCLN early following infection. In marked contrast, IL-17 protein is not detectable by ELISA within dCLN (data not shown), and is present at extremely low levels (≤ 10 pg/mL) within the brains of infected mice. Therefore, the lineage fate of T cells must be decided very early following infection, allowing for MHV-specific T cells to generate a predominant Th-1 response for optimal protection. What remains to be determined is identifying the key signaling events that regulate T-cell differentiation in response to MHV infection, as our findings suggest that neither IL-23 nor IL-12 is essential in this process. Presumably, soluble elements provided by antigen-presenting cells are necessary in tailoring T-cell commitment to a specific lineage. For instance, IL-18 production by antigen-presenting cells can work synergistically with IL-12 to facilitate IFN-γ induction (43). However, this stimulation may not be prominent for viral pathogens, as pulmonary adenovirus infection studies reveal a Th-1 response is still mounted in the absence of both IL-12 and IL-18, despite diminished IFN-γ production (64). Furthermore, the role of TCR signaling strength in relation to IFN-γ induction adds to the complexity of Th-1 commitment (43). Whether the IL-12–independent Th-1 response observed in MHV infection is mediated by TCR antigen-signaling strength or by regulating key T-cell transcription factors such as STAT1 that are necessary for IFN-γ expression, remains to be determined (11,43).
While the functional role of IL-23 in initiating and amplifying autoimmune diseases has been well documented, how IL-23 may regulate immune responses following microbial infection has been less well characterized. Infection of IL-23–deficient mice with Toxoplasma revealed moderate resistance to infection, suggesting that IL-23 is not critical in mounting a protective immune response (35). Respiratory syncytial virus infection of STAT1-deficient animals results in elevated levels of IL-17 that correlated with increased IL-23p19 expression and was accompanied by increased viral burden within the lungs (21). These findings reveal that the absence of the transcription regulator STAT1 amplifies IL-17, yet does not enhance antiviral effector responses by T cells. Vesicular stomatitis virus infection of mice lacking IL-12 receptor beta 1 (IL-12βR1), a shared receptor for IL-12 and IL-23, did not modulate an effective immune response against the virus, suggesting that IL-12/IL-23 are not essential in recovery from viral encephalitis (25). In contrast, infection of mice deficient in IL-12p40, IL-23p19, or IL-17 with Klebsiella pneumoniae resulted in increased mortality, highlighting critical roles for IL-23 and Th-17 cells in pulmonary host defense (18,20). Transient delivery of IL-23 controls growth of Mycobacterium tuberculosis within the lungs of mice that was associated with augmented T-cell responses (19).
Conclusion
Clearly, the dependence on IL-23/IL-17 in providing host defense following infection is controlled by various factors and most likely is dictated by innate immune responses. However, the findings presented in this study provide clear distinction between the functional roles of IL-12/23 signaling pathways in the development of autoimmune inflammatory diseases versus protective antiviral T-cell responses. Importantly, the data presented in this paper suggest that therapeutic strategies designed to inhibit IL-12 and/or IL-23 for treatment of various human autoimmune diseases may not significantly impair the generation of functional virus-specific T cells (26,28,38).
Acknowledgments
This work was supported by National Institutes of Health grants NS41249 and NS18146, and National Multiple Sclerosis Society grant RG3278 to T.E.L. The authors would like to acknowledge and thank Paul Marsters (Centocor) for biostatistical analysis of EAE studies.
References
- 1.Bergmann CC. Lane TE. Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann CC. Parra B. Hinton DR. Chandran R. Morrison M. Stohlman SA. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J Immunol. 2003;170:3204–3213. doi: 10.4049/jimmunol.170.6.3204. [DOI] [PubMed] [Google Scholar]
- 3.Boelen A. Kwakkel J. Barends M. de Rond L. Dormans J. Kimman T. Effect of lack of interleukin-4, interleukin-12, interleukin-18, or the interferon-gamma receptor on virus replication, cytokine response, and lung pathology during respiratory syncytial virus infection in mice. J Med Virol. 2002;66:552–560. doi: 10.1002/jmv.2180. [DOI] [PubMed] [Google Scholar]
- 4.Bowman EP. Chackerian AA. Cua DJ. Rationale and safety of anti-interleukin-23 and anti-interleukin-17A therapy. Curr Opin Infect Dis. 2006;19:245–252. doi: 10.1097/01.qco.0000224818.42729.67. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y. Langrish CL. McKenzie B, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantinescu CS. Tani M. Ransohoff RM, et al. Astrocytes as antigen-presenting cells: expression of IL-12/IL-23. J Neurochem. 2005;95:331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- 7.Croxford JL. Olson JK. Miller SD. Epitope spreading and molecular mimicry as triggers of autoimmunity in the Theiler's virus-induced demyelinating disease model of multiple sclerosis. Autoimmun Rev. 2002;1:251–260. doi: 10.1016/s1568-9972(02)00080-0. [DOI] [PubMed] [Google Scholar]
- 8.Cua DJ. Sherlock J. Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 9.Dorman SE. Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11:321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 10.Dorman SE. Uzel G. Roesler J, et al. Viral infections in interferon-gamma receptor deficiency. J Pediatr. 1999;135:640–643. doi: 10.1016/S0022-3476(99)70064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durbin JE. Hackenmiller R. Simon MC. Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 12.El Behi M. Dubucquoi S. Lefranc D. Zephir H. De Seze J. Vermersch P. Prin L. New insights into cell responses involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Lett. 2005;96:11–26. doi: 10.1016/j.imlet.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Finke D. Brinckmann UG. ter Meulen V. Liebert UG. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass WG. Chen BP. Liu MT. Lane TE. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002;15:261–272. doi: 10.1089/08828240260066215. [DOI] [PubMed] [Google Scholar]
- 15.Glass WG. Hickey MJ. Hardison JL. Liu MT. Manning JE. Lane TE. Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol. 2004;172:4018–4025. doi: 10.4049/jimmunol.172.7.4018. [DOI] [PubMed] [Google Scholar]
- 16.Glass WG. Lane TE. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology. 2003;312:407–414. doi: 10.1016/S0042-6822(03)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez JM. Bergmann CC. Ramakrishna C. Hinton DR. Atkinson R. Hoskin J. Macklin WB. Stohlman SA. Inhibition of interferon-gamma signaling in oligodendroglia delays coronavirus clearance without altering demyelination. Am J Pathol. 2006;168:796–804. doi: 10.2353/ajpath.2006.050496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happel KI. Dubin PJ. Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Happel KI. Lockhart EA. Mason CM. Porretta E. Keoshkerian E. Odden AR. Nelson S. Ramsay AJ. Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs. Infect Immun. 2005;73:5782–5788. doi: 10.1128/IAI.73.9.5782-5788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Happel KI. Zheng M. Young E, et al. Cutting edge: roles of toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–6443. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto K. Durbin JE. Zhou W, et al. Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J Allergy Clin Immunol. 2005;116:550–557. doi: 10.1016/j.jaci.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Heise MT. Virgin HWT. The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Held KS. Chen BP. Kuziel WA. Rollins BJ. Lane TE. Differential roles of CCL2 and CCR2 in host defense to coronavirus infection. Virology. 2004;329:251–260. doi: 10.1016/j.virol.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houtman JJ. Fleming JO. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J Neurovirol. 1996;2:101–110. doi: 10.3109/13550289609146543. [DOI] [PubMed] [Google Scholar]
- 25.Ireland DD. Palian BM. Reiss CS. Interleukin (IL)-12 receptor beta1 or IL-12 receptor beta 2 deficiency in mice indicates that IL-12 and IL-23 are not essential for host recovery from viral encephalitis. Viral Immunol. 2005;18:397–402. doi: 10.1089/vim.2005.18.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauffman CL. Aria N. Toichi E, et al. A phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasis. J Invest Dermatol. 2004;123:1037–1044. doi: 10.1111/j.0022-202X.2004.23448.x. [DOI] [PubMed] [Google Scholar]
- 27.King IL. Segal BM. Cutting edge: IL-12 induces CD4+CD25- T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–645. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 28.Krueger GG. Langley RG. Leonardi C. Yeilding N. Guzzo C. Wang Y. Dooley LT. Lebwohl M. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 29.Lane TE. Fox HS. Buchmeier MJ. Inhibition of nitric oxide synthase-2 reduces the severity of mouse hepatitis virus-induced demyelination: implications for NOS2/NO regulation of chemokine expression and inflammation. J Neurovirol. 1999;5:48–54. doi: 10.3109/13550289909029745. [DOI] [PubMed] [Google Scholar]
- 30.Lane TE. Liu MT. Chen BP, et al. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langrish C L. Chen Y. Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J. Gran B. Zhang GX. Ventura ES. Siglienti I. Rostami A. Kamoun M. Differential expression and regulation of IL-23 and IL-12 subunits and receptors in adult mouse microglia. J Neurol Sci. 2003;215:95–103. doi: 10.1016/s0022-510x(03)00203-x. [DOI] [PubMed] [Google Scholar]
- 33.Li Q. Eppolito C. Odunsi K. Shrikant PA. IL-12-programmed long-term CD8+ T cell responses require STAT4. J Immunol. 2006;177:7618–7625. doi: 10.4049/jimmunol.177.11.7618. [DOI] [PubMed] [Google Scholar]
- 34.Li Y. Chu N. Hu A. Gran B. Rostami A. Zhang GX. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain. 2007;130:490–501. doi: 10.1093/brain/awl273. [DOI] [PubMed] [Google Scholar]
- 35.Lieberman LA. Cardillo F. Owyang AM. Rennick DM. Cua DJ. Kastelein RA. Hunter CA. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol. 2004;173:1887–1893. doi: 10.4049/jimmunol.173.3.1887. [DOI] [PubMed] [Google Scholar]
- 36.Liu MT. Keirstead HS. Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- 37.MacLennan C. Fieschi C. Lammas DA, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190:1755–1757. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 38.Mannon PJ. Fuss IJ. Mayer L, et al. Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med. 2004;351:2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 39.Marten NW. Stohlman SA. Bergmann CC. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 2001;14:1–18. doi: 10.1089/08828240151061329. [DOI] [PubMed] [Google Scholar]
- 40.Marten NW. Stohlman SA. Zhou J. Bergmann CC. Kinetics of virus-specific CD8+ -T-cell expansion and trafficking following central nervous system infection. J Virol. 2003;77:2775–2778. doi: 10.1128/JVI.77.4.2775-2778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mescher MF. Curtsinger JM. Agarwal P. Casey KA. Gerner M. Hammerbeck CD. Popescu F. Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 42.Murphy CA. Langrish CL. Chen Y. Blumenschein W. Mc-Clanahan T. Kastelein RA. Sedgwick JD. Cua DDJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nembrini C. Abel B. Kopf M. Marsland BJ. Strong TCR signaling, TLR ligands, and cytokine redundancies ensure robust development of type 1 effector T cells. J Immunol. 2006;176:7180–7188. doi: 10.4049/jimmunol.176.12.7180. [DOI] [PubMed] [Google Scholar]
- 44.Novelli F. Casanova JL. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine Growth Factor Rev. 2004;15:367–377. doi: 10.1016/j.cytogfr.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oppmann B. Lesley R. Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 46.Parham C. Chirica M. Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 47.Parra B. Hinton DR. Lin MT. Cua DJ. Stohlman SA. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology. 1997;233:260–270. doi: 10.1006/viro.1997.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra B. Hinton DR. Marten NW. Bergmann CC. Lin MT. Yang CS. Stohlman SA. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- 49.Rice L. Orlow D. Ceonzo K. Stahl GL. Tzianabos AO. Wada H. Aird WC. Buras JA. CpG oligodeoxynucleotide protection in polymicrobial sepsis is dependent on interleukin-17. J Infect Dis. 2005;191:1368–1376. doi: 10.1086/428452. [DOI] [PubMed] [Google Scholar]
- 50.Sato K. Suematsu A. Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schijns VE. Haagmans BL. Wierda CM. Kruithof B. Heijnen IA. Alber G. Horzinek MNC. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J Immunol. 1998;160:3958–3964. [PubMed] [Google Scholar]
- 52.Schijns VE. Wierda CM. van Hoeij M. Horzinek MC. Exacerbated viral hepatitis in IFN-gamma receptor-deficient mice is not suppressed by IL-12. J Immunol. 1996;157:815–821. [PubMed] [Google Scholar]
- 53.Shevach EM. Chang JT. Segal BM. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin Immunopathol. 1999;21:249–262. doi: 10.1007/BF00812256. [DOI] [PubMed] [Google Scholar]
- 54.Sonobe Y. Yawata I. Kawanokuchi J. Takeuchi H. Mizuno T. Suzumura A. Production of IL-27 and other IL-12 family cytokines by microglia and their subpopulations. Brain Res. 2005;1040:202–207. doi: 10.1016/j.brainres.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 55.Stohlman SA. Bergmann CC. Lin MT. Cua DJ. Hinton DR. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 56.Stohlman SA. Hinton DR. Cua D. Dimacali E. Sensintaffar J. Hofman FM. Tahara SM. Yao Q. Tumor necrosis factor expression during mouse hepatitis virus-induced demyelinating encephalomyelitis. J Virol. 1995;69:5898–5903. doi: 10.1128/jvi.69.9.5898-5903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theiner G. Rossner S. Dalpke A. Bode K. Berger T. Gessner A. Lutz MB. TLR9 cooperates with TLR4 to increase IL-12 release by murine dendritic cells. Mol Immunol. 2008;45:244–252. doi: 10.1016/j.molimm.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Trifilo MJ. Lane TE. The CC chemokine ligand 3 regulates CD11c+CD11b+CD8alpha- dendritic cell maturation and activation following viral infection of the central nervous system: implications for a role in T cell activation. Virology. 2004;327:8–15. doi: 10.1016/j.virol.2004.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe R. Wege H. ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983;305:150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu GF. Dandekar AA. Pewe L. Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- 61.Wu GF. Dandekar AA. Pewe L. Perlman S. The role of CD4 and CD8 T cells in MHV-JHM-induced demyelination. Adv Exp Med Biol. 2001;494:341–347. doi: 10.1007/978-1-4615-1325-4_51. [DOI] [PubMed] [Google Scholar]
- 62.Wu GF. Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J Virol. 1999;73:8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Q. Martin RJ. Rino JG. Breed R. Torres RM. Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xing Z. Zganiacz A. Wang J. Divangahi M. Nawaz F. IL-12-independent Th1-type immune responses to respiratory viral infection: requirement of IL-18 for IFN-gamma release in the lung but not for the differentiation of viral-reactive Th1-type lymphocytes. J Immunol. 2000;164:2575–2584. doi: 10.4049/jimmunol.164.5.2575. [DOI] [PubMed] [Google Scholar]
- 65.Zheng Y. Danilenko DM. Valdez P. Kasman I. Eastham-Anderson J. Wu J. Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]