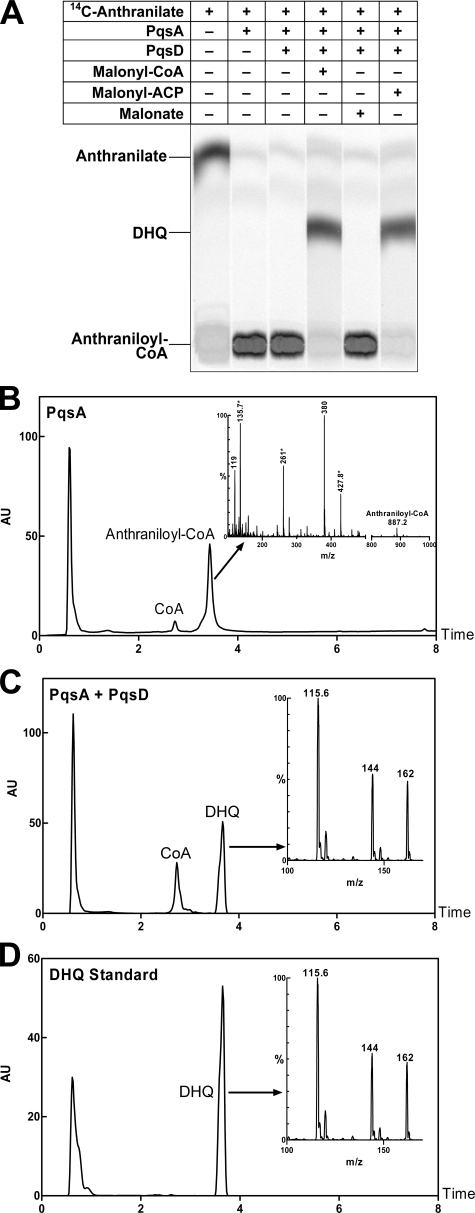
FIGURE 3.
PqsD catalyzes the formation of DHQ in vitro. A, TLC analysis of PqsD reaction products showed that both malonyl-CoA and malonyl-ACP were substrates, whereas malonic acid was not. The reactants are indicated above each lane. The identity of the PqsD reaction product was determined by LC-MS analyses (B–D). (Each panel shows the liquid chromatograph with the mass spectrum of the indicated peak shown in the inset.) B, anthraniloyl-CoA was the product of PqsA. Anthraniloyl-CoA was eluted at 3.43 min, and the protonated molecular ion (M + H+) at m/z 887.2 was present. The ions indicated by asterisks were derived from CoA. The ion at m/z 119 was the anthraniloyl moiety; the ion at m/z 380 was the anthraniloyl moiety plus the CoA ion at m/z 261. AU, absorbance units. C, DHQ and CoA were the products of the PqsA-plus-PqsD reaction. D, shown are the results from LC-MS of the DHQ standard. DHQ was eluted at 3.67 min; the molecular ion of DHQ was at m/z 162. The fragment ions at m/z 144 and 115 are characteristic of DHQ (13).