FIGURE 4.
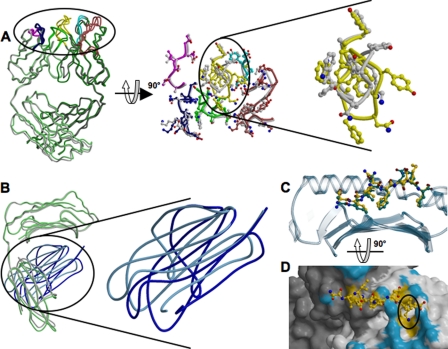
Structural changes of 25-D1.16 and pOV8-Kb upon specific complex formation. A, the left panel shows the superposition of intact (green) and pOV8-Kb-bound (gray) 25-D1.16 Fab. CDRs for both structures are colored as described in the legend to Fig. 1A. The middle panel shows a close-up view of the CDRs oriented to reveal the pMHC-binding interface. Gray CDRs correspond to the isolated Fab fragment, and the colored CDRs correspond to pOV8-Kb-bound Fab. The right panel is a close-up view of CDR-H3 and reveals the conformational change induced upon binding of Fab to pOV8-Kb. B, the right panel shows the superposition of isolated MHC (with the heavy chain colored gray and β2m colored cyan) and pOV8-Kb-bound MHC (with the heavy chain colored green and β2m colored blue). The left panel shows a close-up view of the two β2m domains, revealing distinct orientations. C, the pOV8 peptide as bound to intact Kb (with carbon atoms colored green) and in the complex with 25-D1.16 (with carbon atoms colored yellow) is shown. The polymorphic domain is shown as a blue transparent ribbon. The peptide is pulled out by the bound Fab fragment. D, shown are the molecular surface of 25-D1.16 and a ball-and-stick diagram of the MHC-bound peptide. Fab atoms are colored according to their contacts (surface contacts MHC (blue) and surface contacts the peptide (yellow)). LysP7 (highlighted by the black circle) is enveloped by CDR-H3.