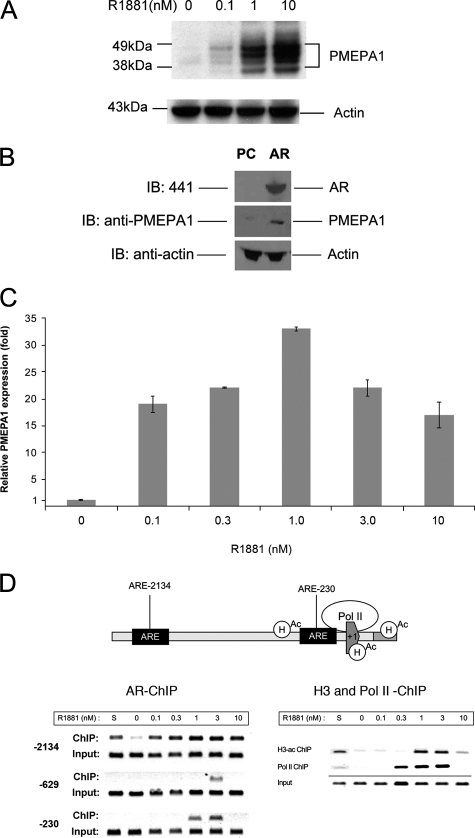
FIGURE 1.
PMEPA1 expression is regulated by AR. A, LNCaP cells were maintained in hormone-depleted (cFBS) media for 5 days followed by the addition of indicated doses of R1881 synthetic androgen for 48 h. Thirty micrograms of cell lysates were subjected to 4–12% of NuPAGE and immunoblotted with anti-PMEPA1 monoclonal antibody 2A12 and anti-actin antibody. Molecular mass markers are shown in kDa. B, COS-7 cells were transfected with pcDNA3.1 (PC) or AR-pcDNA3.1 (AR) expression vectors under the control of cytomegalovirus promoter. After incubation for 24 h cell lysates were analyzed by immunoblot assays (IB) with anti-AR (441), anti-PMEPA1, and anti-actin antibodies visualizing AR (100 kDa), multiple forms of PMEPA1 (32–40 kDa) and actin (34 kDa) proteins, respectively. C and D, LNCaP cells were grown in hormone-depleted cFBS-containing media for 5 days followed either by the addition of 0, 0.1, 0.3, 1.0, 3.3, 10 nm of R1881 or by the addition of FBS containing media (S). Cells were incubated for 24 h, and the expression of PMEPA1 was analyzed by quantitative PCR. PMEPA1 expression fold change was normalized to the levels of the housekeeping gene GAPDH. PMEPA1 expression is shown relative to the levels in hormone-depleted (cFBS, 0 nm R1881) LNCaP cells (bars equal mean ± S.E. (n = 3)). In semi-quantitative chromatin immunoprecipitation assay anti-AR, anti-RNA polymerase II or anti-acetylated H3 histone antibodies were used. Specific primer pairs were selected for amplifying the –2134, –230, and the low affinity –629 AREs within the PMEPA1 promoter upstream sequences. To assay the core promoter for RNA polymerase II (polII) and acetyl histone H3 (H3-ac) recruitment the +81 and +199 were amplified. To achieve linear amplifications ranges the ChIP and input products were amplified with 38 and 33 cycles, respectively.